Alternatived Products of [ 2437-42-5 ]
Product Details of [ 2437-42-5 ]
| CAS No. : | 2437-42-5 |
MDL No. : | MFCD02665277 |
| Formula : |
C11H11N
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
157.21
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 2437-42-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2437-42-5 ]
- 1
-
 [ 110-00-9 ]
[ 110-00-9 ]

-
 [ 95-53-4 ]
[ 95-53-4 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
- 2
-
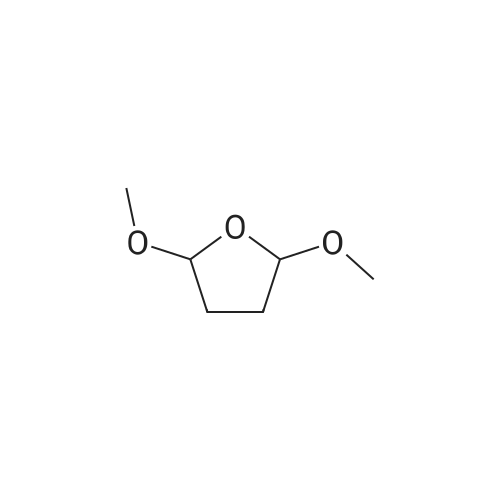 [ 696-59-3 ]
[ 696-59-3 ]

-
 [ 95-53-4 ]
[ 95-53-4 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 90% |
With L-(+)-tartaric acid-choline chloride based deep eutectic solvent; at 90℃; for 0.666667h;Green chemistry; |
General procedure: Amine (1 mmol), 2,5-dimethoxytetrahydrofuran (1.1 mmol)and L-(+)-tartaric acid-choline chloride based DES (1.5 g) were added to a 50 mL round bottom flask and the reaction mixturewas stirred at 90 C. The progress of the reaction was monitoredby TLC. After completion of the reaction, the mixture was cooled to room temperature and the product was extracted with ethyl acetate.After the evaporation of the solvent, the residue was purified by columnchromatography on silica gel to afford the pure product. The DES wasdried under vacuumand reused for the next cycle. |
| 89% |
With ionic liquid immobilized on gamma-Fe2O3(at)SiO2 nanoparticles; In water; at 100℃; for 0.666667h; |
General procedure: To a solution of amine (1 mmol) in water (2 ml) was added tetrahydro-2,5-dimethoxyfuran (1.1 mmol) and gamma-Fe2O3(at)SiO2-Sb-IL (0.08 g). The reaction mixture was stirred at 100 C for a certain period of time as required to complete the reaction. During that time, the reaction was monitored constantly by TLC. After completion of the reaction, the catalyst was removed by using a magnet and washed with ethyl acetate. The aqueous solution was extracted by ethyl acetate (3 × 5 ml). The combined organic phase was dehydrated with anhydrous sodium sulfate. After the evaporation of the solvent, the residue was purified by silica gel flash chromatography using petroleum ether/ethyl acetate as the eluent to afford the pure product. |
| 84% |
With magnesium iodide etherate; In acetonitrile; at 80℃; for 6h; |
General procedure: A Schlenk reaction tube was charged with primary aromatic amine (5.0 mmol), 2,5-dimethoxytetrahydrofuran (6.0 mmol), MgI2 etherate (10% mmol), and acetonitrile (10 mL). The reaction mixture was stirred at 80 C for several hours and then concentrated in vacuo. The residue was purified by flash column chromatography on a silica gel to give the desired product. |
Reference:
[1]Tetrahedron Letters,2009,vol. 50,p. 4807 - 4809
[2]Synlett,2009,p. 2245 - 2248
[3]Monatshefte fur Chemie,2013,vol. 144,p. 405 - 409
[4]Journal of Molecular Liquids,2014,vol. 198,p. 259 - 262
[5]Applied Catalysis A: General,2013,vol. 457,p. 34 - 41
[6]Journal of Chemical Research,2009,p. 14 - 16
[7]Tetrahedron,2011,vol. 67,p. 898 - 903
[8]Bioorganic and Medicinal Chemistry Letters,2005,vol. 15,p. 3753 - 3757
[9]Journal of Heterocyclic Chemistry,2019,vol. 56,p. 1337 - 1340
[10]Journal of Medicinal Chemistry,2014,vol. 57,p. 6531 - 6552
[11]Acta Chemica Scandinavica (1947),1952,vol. 6,p. 867,872
- 3
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 42824-16-8 ]
[ 42824-16-8 ]

-
1-<i>o</i>-tolyl-pyrrole-2-sulfonic acid
[ No CAS ]
- 4
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 42824-16-8 ]
[ 42824-16-8 ]

-
 [ 107-06-2 ]
[ 107-06-2 ]

-
1-<i>o</i>-tolyl-pyrrole-2-sulfonic acid
[ No CAS ]
- 5
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
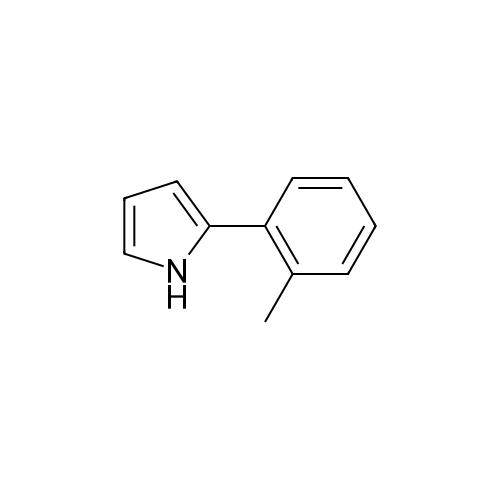 [ 368212-31-1 ]
[ 368212-31-1 ]
- 6
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
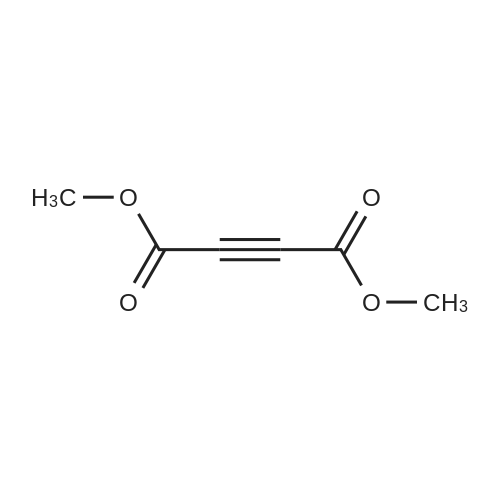 [ 762-42-5 ]
[ 762-42-5 ]

-
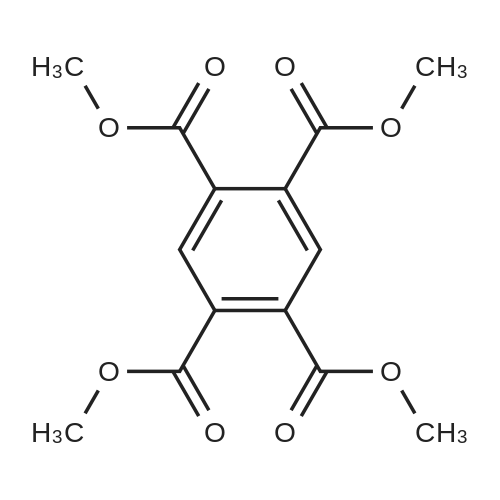 [ 635-10-9 ]
[ 635-10-9 ]
- 7
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 762-42-5 ]
[ 762-42-5 ]

-
 [ 74986-15-5 ]
[ 74986-15-5 ]

-
 [ 74986-03-1 ]
[ 74986-03-1 ]

-
 [ 74965-14-3 ]
[ 74965-14-3 ]
- 8
-
 [ 696-59-3 ]
[ 696-59-3 ]

-
 [ 95-53-4 ]
[ 95-53-4 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 41378-30-7 ]
[ 41378-30-7 ]
- 10
-
mucate o-toluidine
[ No CAS ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
- 11
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 124-38-9 ]
[ 124-38-9 ]

-
 [ 55540-39-1 ]
[ 55540-39-1 ]

-
 [ 35524-49-3 ]
[ 35524-49-3 ]
- 12
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 124-38-9 ]
[ 124-38-9 ]

-
 [ 35524-49-3 ]
[ 35524-49-3 ]

-
1-(2-carboxymethyl-phenyl)-1<i>H</i>-pyrrole-2-carboxylic acid
[ No CAS ]
- 13
-
 [ 109-97-7 ]
[ 109-97-7 ]

-
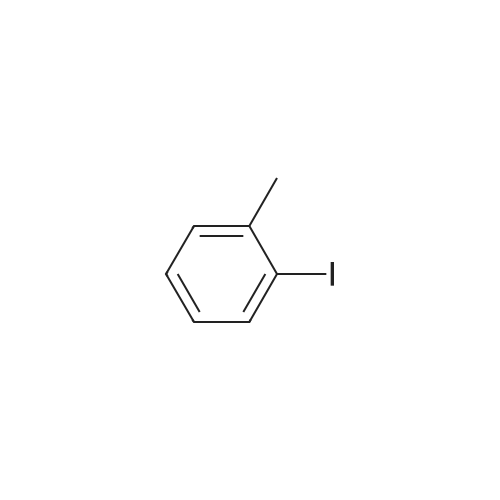 [ 615-37-2 ]
[ 615-37-2 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 37% |
With copper(ll) sulfate pentahydrate; sodium hydroxide; In dimethyl sulfoxide; at 110℃; for 12h;Sealed tube; |
General procedure: CuSO4·5H2O (12.50 mg, 0.05 mmol), the aryl iodide or bromide(1.0 mmol), pyrrole (1.5 mmol), NaOH (80 mg, 2 mmol), and DMSO(2 mL) were placed in a 10 mL sealed tube. The mixture was heatedat 110 C in a preheated oil bath for 12 h. It was then cooled to roomtemperature, diluted with 20 mL H2O, and the mixture was extractedwith ethyl acetate (3 × 20 mL). The combined organic phases waswashed with water and brine, dried over anhydrous Na2SO4, andconcentrated in vacuo. The residue was purified by flash columnchromatography on silica gel (ethyl acetate/petroleum ether, 1 : 100)to afford the target products. All C-N coupling products reported hereare known products and were characterised by GC-MS and 1H NMR,which were compared with the previously reported dates. |
- 14
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 55962-05-5 ]
[ 55962-05-5 ]

-
3,4-di(toluene-p-sulfonamido)-1-(2-methylphenyl)pyrrole
[ No CAS ]
- 15
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 68-12-2 ]
[ 68-12-2 ]

-
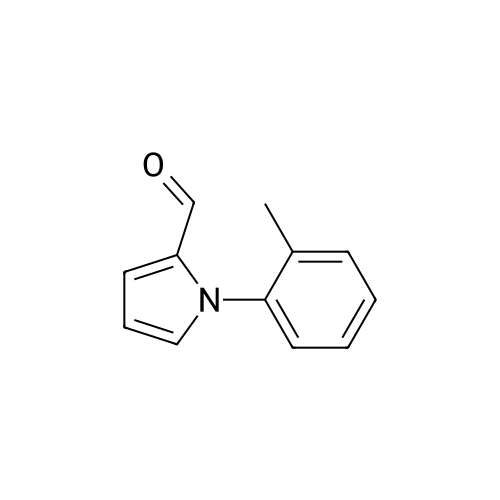 [ 35524-41-5 ]
[ 35524-41-5 ]

-
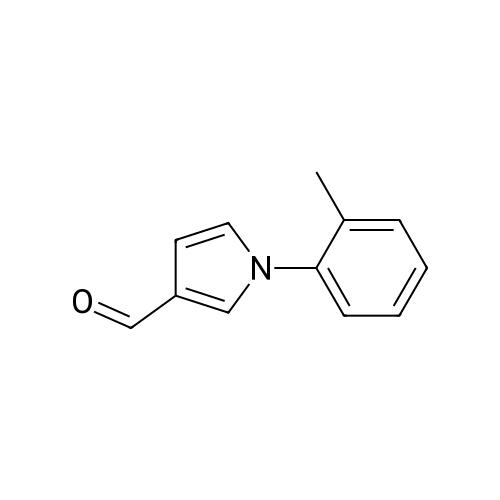 [ 864547-96-6 ]
[ 864547-96-6 ]
- 16
-
 [ 109-97-7 ]
[ 109-97-7 ]

-
 [ 95-46-5 ]
[ 95-46-5 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
- 17
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 143-33-9 ]
[ 143-33-9 ]

-
 [ 35524-47-1 ]
[ 35524-47-1 ]
- 18
-
 [ 109-97-7 ]
[ 109-97-7 ]

-
 [ 95-53-4 ]
[ 95-53-4 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
- 19
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
1-(2-methoxy-phenyl)-4-(1-<i>o</i>-tolyl-1<i>H</i>-pyrrol-3-ylmethyl)-piperazine
[ No CAS ]
- 20
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
1-(2-methoxy-phenyl)-4-(1-<i>o</i>-tolyl-1<i>H</i>-pyrrol-2-ylmethyl)-piperazine
[ No CAS ]
- 21
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
3-[4-(1-<i>o</i>-tolyl-1<i>H</i>-pyrrol-2-ylmethyl)-piperazin-1-yl]-benzo[<i>d</i>]isoxazole
[ No CAS ]
- 22
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
3-[4-(1-<i>o</i>-tolyl-1<i>H</i>-pyrrol-3-ylmethyl)-piperazin-1-yl]-benzo[<i>d</i>]isoxazole
[ No CAS ]
- 23
-
 [ 696-59-3 ]
[ 696-59-3 ]

-
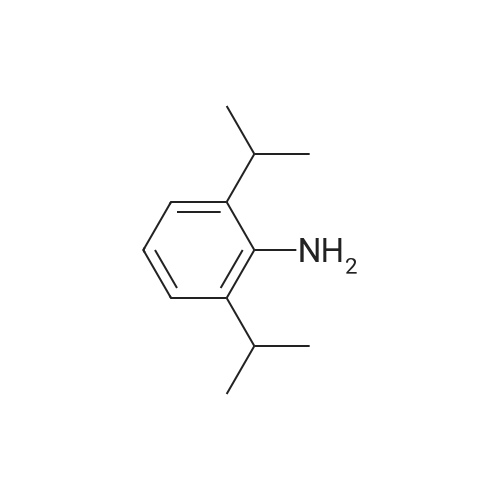 [ 24544-04-5 ]
[ 24544-04-5 ]

-
 [ 95-53-4 ]
[ 95-53-4 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
1-(2,6-diisopropylphenyl)-1H-pyrrole
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With magnesium iodide etherate; In acetonitrile; at 80℃; |
General procedure: A Schlenk reaction tube was charged with each amine (5.0 mmol), 2,5-dimethoxytetrahydrofuran (6.0 mmol), MgI2 etherate (10% mmol), and acetonitrile (10 mL). The reaction mixture was stirred at 80 C for several hours and then concentrated in vacuo. Flash column chromatography afforded the desired products. The ratio of each product was determined by column chromatography isolation or GC analysis. |
- 24
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 4497-57-8 ]
[ 4497-57-8 ]

-
 [ 35524-41-5 ]
[ 35524-41-5 ]

-
 [ 864547-96-6 ]
[ 864547-96-6 ]
- 25
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
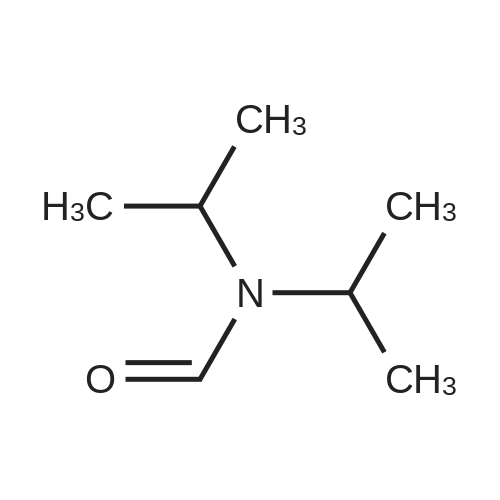 [ 2700-30-3 ]
[ 2700-30-3 ]

-
 [ 35524-41-5 ]
[ 35524-41-5 ]

-
 [ 864547-96-6 ]
[ 864547-96-6 ]
- 26
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 607-00-1 ]
[ 607-00-1 ]

-
 [ 35524-41-5 ]
[ 35524-41-5 ]

-
 [ 864547-96-6 ]
[ 864547-96-6 ]
- 27
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 52008-97-6 ]
[ 52008-97-6 ]

-
 [ 35524-41-5 ]
[ 35524-41-5 ]

-
 [ 864547-96-6 ]
[ 864547-96-6 ]
- 28
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
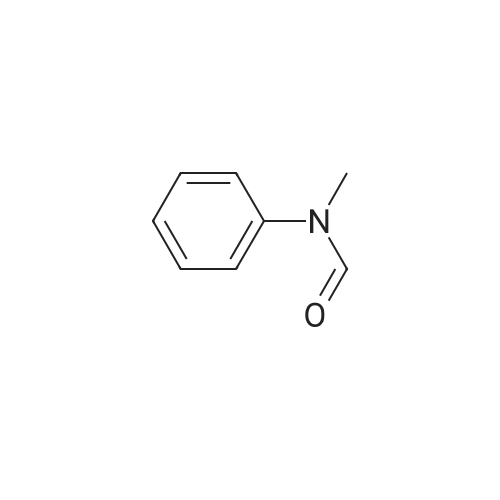 [ 93-61-8 ]
[ 93-61-8 ]

-
 [ 35524-41-5 ]
[ 35524-41-5 ]

-
 [ 864547-96-6 ]
[ 864547-96-6 ]
- 29
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
N-(2,6-dimethylphenyl)-N-methylformamide
[ No CAS ]

-
 [ 35524-41-5 ]
[ 35524-41-5 ]

-
 [ 864547-96-6 ]
[ 864547-96-6 ]
- 30
-
 [ 474094-54-7 ]
[ 474094-54-7 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]
- 31
-
 [ 474094-54-7 ]
[ 474094-54-7 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
1-<i>o</i>-tolyl-2,5-dihydro-pyrrole
[ No CAS ]
- 32
-
 [ 2437-42-5 ]
[ 2437-42-5 ]

-
 [ 4521-61-3 ]
[ 4521-61-3 ]

-
(1-(2-tolyl)-1H-pyrrol-3-yl)(3,4,5-trimethoxyphenyl)methanone
[ No CAS ]
- 33
-
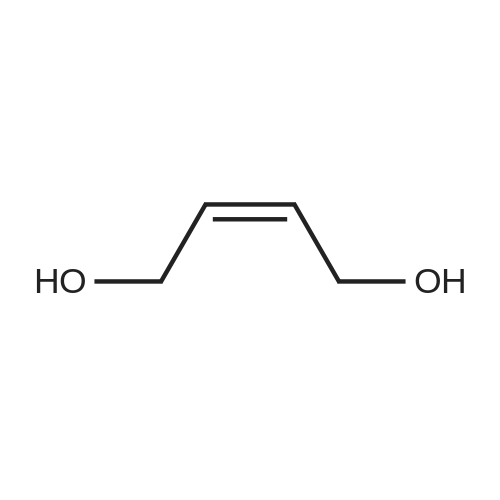 [ 6117-80-2 ]
[ 6117-80-2 ]

-
 [ 95-53-4 ]
[ 95-53-4 ]

-
 [ 2437-42-5 ]
[ 2437-42-5 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping

















