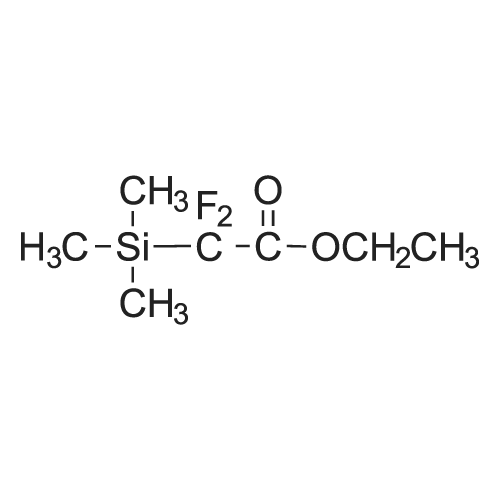Alternatived Products of [ 2740-87-6 ]
Product Details of [ 2740-87-6 ]
| CAS No. : | 2740-87-6 |
MDL No. : | MFCD09025694 |
| Formula : |
C8H6FNS
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
167.20
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 2740-87-6 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 2740-87-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2740-87-6 ]
- 1
-
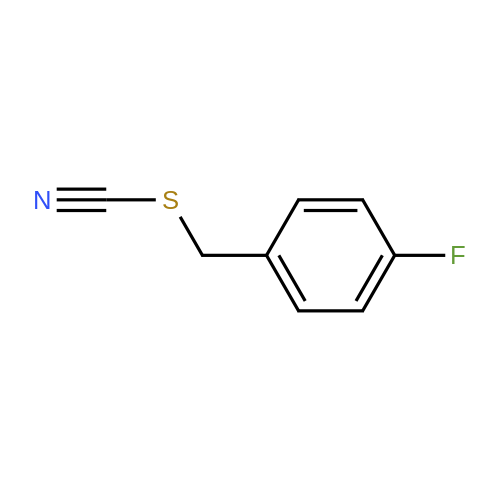 [ 2740-87-6 ]
[ 2740-87-6 ]

-
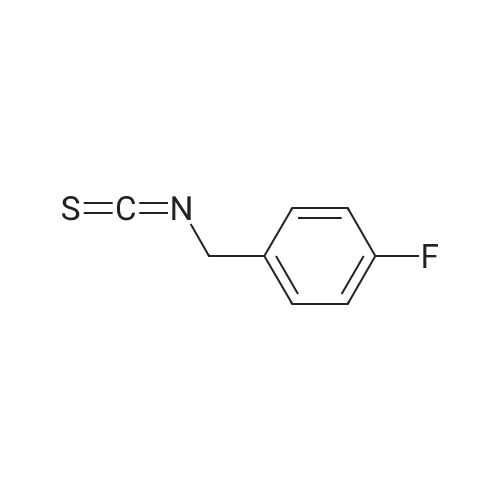 [ 2740-88-7 ]
[ 2740-88-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 9,10-diphenylanthracene In benzene for 2h; Irradiation; |
|
|
With 9,10-diphenylanthracene In benzene Irradiation; |
|
Reference:
[1]Wakamatsu, Kan; Dairiki, Jun; Etoh, Tetsuo; Yamamoto, Hiroshi; Yamamoto, Satoshi; Shigetomi, Yasumasa
[Tetrahedron Letters, 2000, vol. 41, # 3, p. 365 - 369]
[2]Wakamatsu, Kan; Dairiki, Jun; Etoh, Tetsuo; Yamamoto, Hiroshi; Yamamoto, Satoshi; Shigetomi, Yasumasa
[Tetrahedron Letters, 2000, vol. 41, # 3, p. 365 - 369]
- 2
-
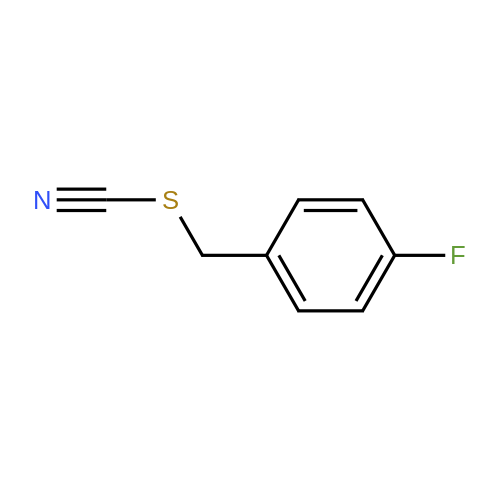 [ 2740-87-6 ]
[ 2740-87-6 ]

-
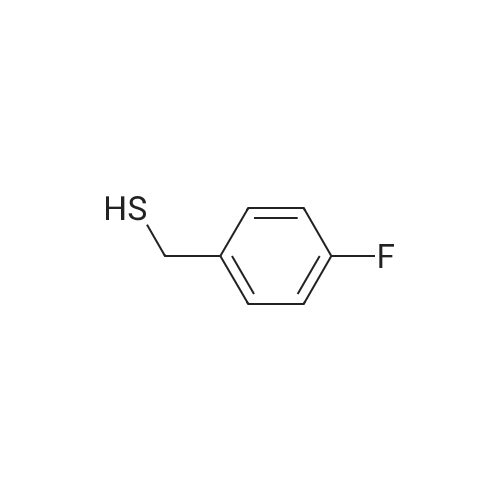 [ 15894-04-9 ]
[ 15894-04-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 75% |
With tetraphosphorus decasulfide In toluene for 3h; Reflux; |
General experimental procedure:
General procedure: General experimental procedure: In a three-neck roundbottom flask, to a solution of thiocyanate (10 mmol) in toluene(25 mL), P2S5 (2.22 g, 10 mmol) was added and the resultingsuspension was refluxed till complete consumption of thestarting material (TLC). After the reaction was complete, thereaction mixture was quenched by careful addition of water(10 mL), extracted with ethyl acetate (3 × 10 mL), the organicphase was dried over sodium sulfate and evaporated underreduced pressure to get the crude product which was purified byflash chromatography (hexane-ethyl acetate) to get the purethiol. |
- 3
-
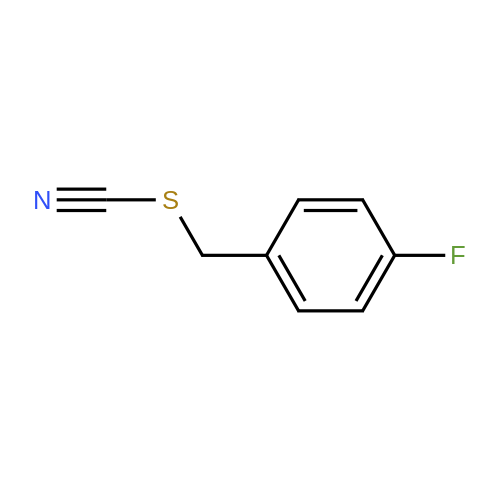 [ 2740-87-6 ]
[ 2740-87-6 ]

-
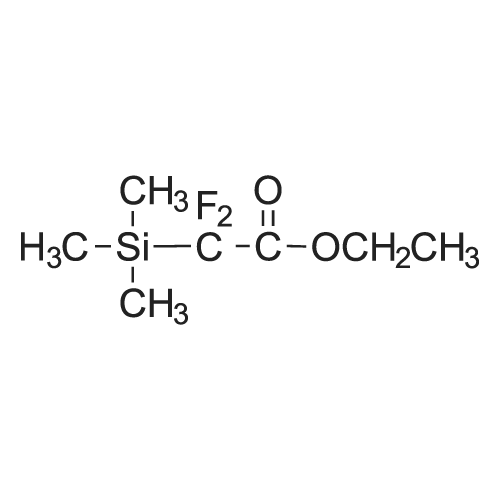 [ 205865-67-4 ]
[ 205865-67-4 ]

-
ethyl 2,2-difluoro-2-((4-fluorobenzyl)thio)acetate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 105.6 mg |
With cesium fluoride In N,N-dimethyl acetamide at 25℃; for 5h; |
4.1 General procedure for the synthesis of derivatives 3 or 5
General procedure: An oven-dried 20mL crimp cap vessel with Teflon-coated stirrer bar was charged with the bromid 1 or 4 (0.5mmol) and NaSCN (1.0mmol) in DMA (5mL), and the suspension was stirred at room temperature for 1h (for 3n-3s: 1 and NaSCN were firstly stirred at 60°C in DMA for 1h then cooled to room temperature). Then, ethyl 2,2-difluoro-2-(trimethylsilyl)acetate (TMS-CF2CO2Et) (1.0mmol) was added to the reaction mixture followed by CsF (1.0mmol) or NaOAc (1.0mmol). The obtained reaction mixture was stirred at 25°C for 5h. The mixture was quenched with water (5mL) and extracted with EtOAc (3×5mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated. After evaporation of the solvent, the residue was purified by flash column chromatography (SiO2, petroleum ether/ethyl acetate=100:1) to afford the desired products 3 or 5. |
- 4
-
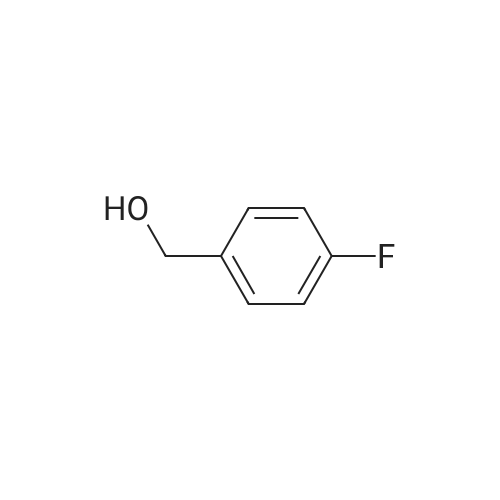 [ 459-56-3 ]
[ 459-56-3 ]

-
 [ 1147550-11-5 ]
[ 1147550-11-5 ]

-
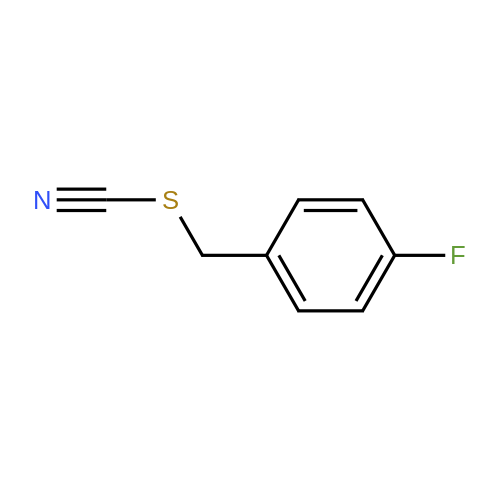 [ 2740-87-6 ]
[ 2740-87-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
With fluorosulfonyl fluoride; sodium carbonate In ethyl acetate at 20℃; for 5h; |
1. General procedure for the SO2F2-promoted thiocyanation of alcohols tothiocyanates 2
General procedure: Alcohol substrates 1a-1ad (1.0 mmol, 1.0 equiv), ammonium thiocyanate (1.0 mmol, 1.0equiv), Na2CO3 (4.0 mmol, 4.0 equiv) and EtOAc (2.0 mL, 0.5 M) were sequentially addedinto an oven-dried reaction tube (30 mL) equipped with a stirring bar, the reaction tube wascovered with a plastic stopper before the SO2F2 gas was introduced into the stirring reactionmixture by slow bubbling through SO2F2 balloon at the room temperature for 5 h. Then, thereaction diluted with water and extracted with ethyl acetate (3× 25 mL). Then the combinedorganic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated todryness. The residue was purified through silica gel chromatography using a mixture of ethylacetate and petroleum ether as eluent to afford the desired benzyl thiocyanates 2a-2ag. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping