Alternatived Products of [ 2866-43-5 ]
Product Details of [ 2866-43-5 ]
| CAS No. : | 2866-43-5 |
MDL No. : | MFCD00205000 |
| Formula : |
C18H10N2O2S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
318.35
|
Pubchem ID : | - |
| Synonyms : |
|
Chemical Name : | 2,5-Bis(benzo[d]oxazol-2-yl)thiophene |
Application In Synthesis of [ 2866-43-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2866-43-5 ]
- 1
-
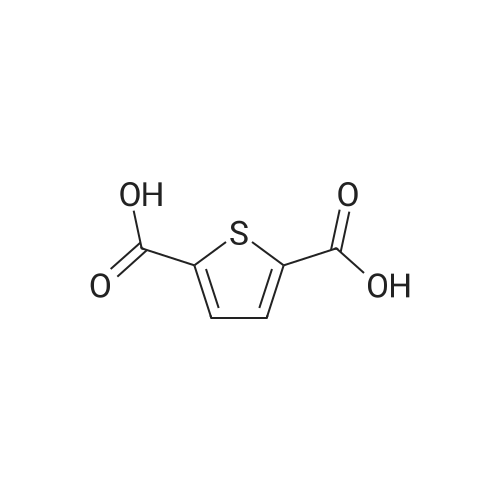 [ 4282-31-9 ]
[ 4282-31-9 ]

-
 [ 95-55-6 ]
[ 95-55-6 ]

-
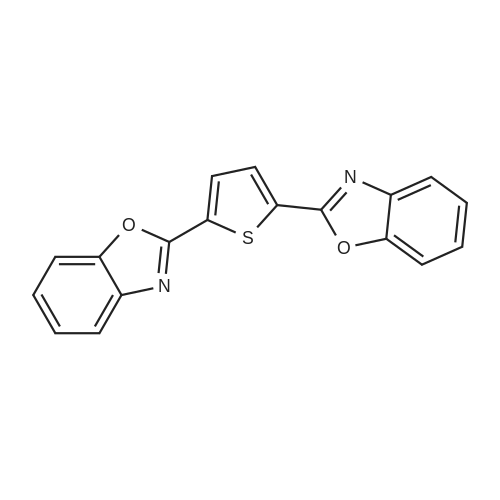 [ 2866-43-5 ]
[ 2866-43-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 5.5% |
|
A solution of amino compound (XV) (5.8 mmoles) and 2,5- thiophenedicarboxylic acid (5.8 mmoles) in PPA (10 ml) was stirred under N2 atmosphere at 150 C for 2h. Cooled the reaction mass to RT, poured into cold water, neutralized with ammonia solution to pH~ 10-1 1, then extracted with ethyl acetate, washed with brine followed by water, dried the organic layer over anhydrous Na2SO4 and distilled to give the crude product, which was then purified by using silica gel 60-120 mesh column chromatography and DCM: methanol 100-5 % as mobile phase to get the pure compound (XX). Table 11: preparation of thiophene benzimidazole dimer (XX). |
|
With boric acid; In 1-methyl-pyrrolidin-2-one; toluene; at 20 - 185℃; for 13h; |
200 g of N-methylpyrrolidone are charged to a reaction vessel and 52 g of 98% thiophene-2,5-dicarboxylic acid, followed by 72 g of 99% 2-aminophenol, 10 g of boric acid and 30 g of toluene are added with stirring. The apparatus, equipped with a Dean and Stark water trap, is evacuated and the vacuum released with nitrogen. The light yellow suspension is heated to 185[deg.] C. and stirred at this temperature for 12 hours, during which time 23-25 ml of water and approximately 25 g of toluene are distilled off through the water trap. The reaction mixture is cooled to 20[deg.] C. and stirring continued for 1 hour at this temperature. The yellow suspension is filtered, washed with 100 g of N-methylpyrrolidone to give 300 g of a brown solution which may be used as solvent for a further charge and then with three 80 g portions of water. The resulting press-cake is dried under a vacuum of 50 mbar at 100[deg.] C. to yield 75 g of the compound of formula (104) as a yellow solid, characterized by a UV absorption maximum [lambda]max at 372 nm with an extinction coefficient [epsilon] of 52000 and by the following <1>H-NMR data in D6-DMSO: [0045] 8.10, 2H, s; 7.82, 4H, m and 7.50, 4H, m. |
- 2
-
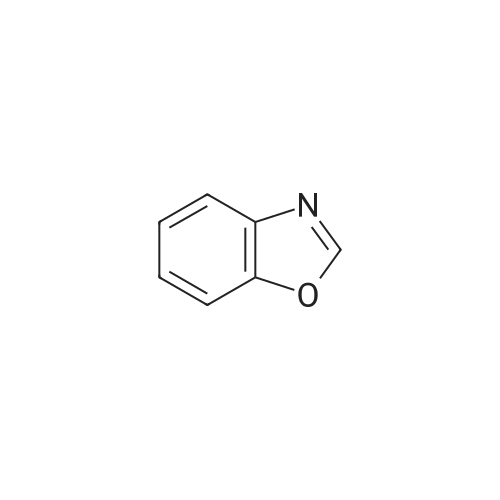 [ 273-53-0 ]
[ 273-53-0 ]

-
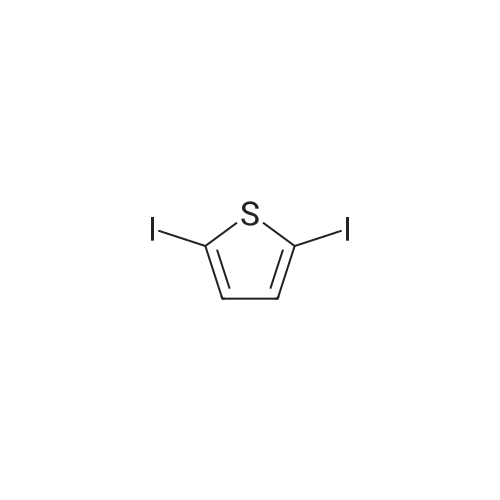 [ 625-88-7 ]
[ 625-88-7 ]

-
 [ 2866-43-5 ]
[ 2866-43-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 89% |
With [Pd(1,10-phenanthroline)2](PF6)2; caesium carbonate In dimethyl amine at 150℃; for 20h; Inert atmosphere; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








