Alternatived Products of [ 287118-98-3 ]
Product Details of [ 287118-98-3 ]
| CAS No. : | 287118-98-3 |
MDL No. : | N/A |
| Formula : |
C16H14O5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
286.28
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 287118-98-3 ]
Application In Synthesis of [ 287118-98-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 287118-98-3 ]
- 1
-
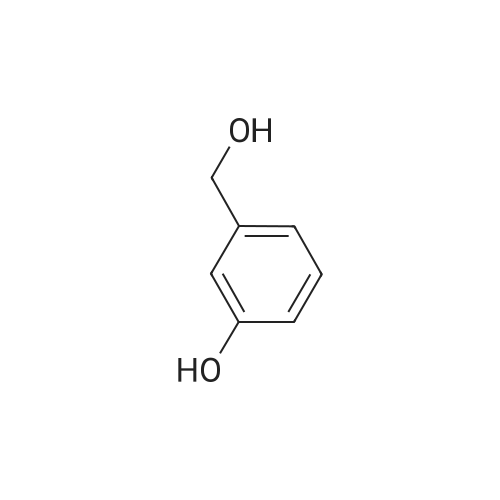 [ 620-24-6 ]
[ 620-24-6 ]

-
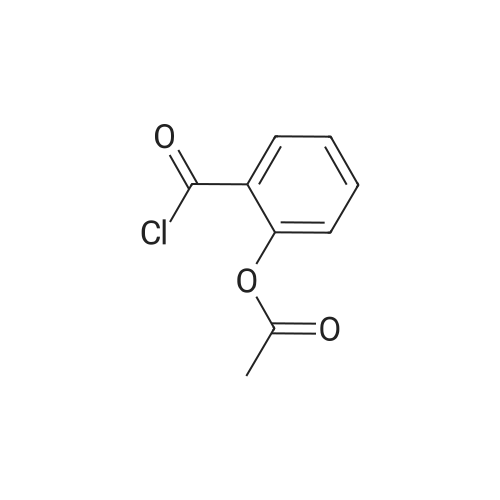 [ 5538-51-2 ]
[ 5538-51-2 ]

-
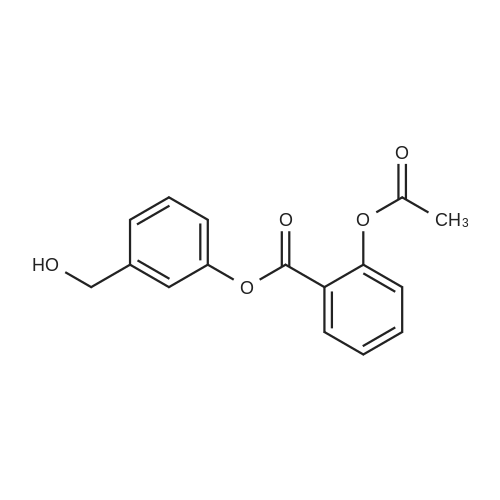 [ 287118-98-3 ]
[ 287118-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 91% |
With potassium carbonate In acetone at 5 - 10℃; for 2h; |
1c Example 1c; Preparation of 3-Hydroxymethylphenyl Ester of the 2-Acetoxybenzoic Acid (Compound I-B) in Organic Solvent Miscible With Water
3-hydroxymethylphenol (10 g, 0.08 moles) is dissolved in acetone (50 ml). In the obtained solution potassium carbonate in powder (22.2 g, 0.16 moles) is suspended. To the suspension an acetylsalicylic acid chloride solution (16 g, 0.08 moles) in acetone (50 ml) is added at a temperature of 5°-10° C. under stirring. The mixture is maintained at a temperature in the above mentioned range, under stirring, for 2 hours, then filtered and the solvent evaporated under vacuum. The residue is crystallized from isopropanol. 3-hydroxymethylphenyl ester of the 2-acetoxy-benzoic acid (21.0 g, 0.07 moles, yield 91%) is obtained. [00043] M.P.: 79°-80° C. 1H NMR(CDCl3) δ (ppm): 2.29 (s, 3H); 4.71 (s, 2H); 7.07-8.2 (m, aromatics, 8H). |
| 80% |
With sodium hydroxide In dichloromethane; water at 20℃; for 2h; |
1a Example 1a; Preparation of 3-Hydroxymethylphenyl Ester of the 2-Acetoxybenzoic Acid (Compound I-B) in Admixture Water-organic Solvent
3-hydroxymethylphenol (25.25 g, 0.2 moles) is dissolved in a 5% hydroxide sodium solution (160 ml). To the so obtained solution an acetylsalicylic acid chloride solution (40.4 g, 0.2 moles) in dichloromethane (50 ml) is added at roam temperature, under stirring. The mixture is maintained at room temperature under stirring for 2 hours and then extracted with dichloromethane (2×100 ml). The organic phase is separated, anhydrified with sodium sulphate and the solvent evaporated under vacuum. The residue is crystallized from a mixture of ethyl acetate and hexane. 3-hydroxymethylphenyl ester of the 2-acetoxybenzoic acid (45.8 g, 0.16 moles, yield 80%) is obtained. [00039] M.P.: 79°-81° C. 1H NMR(CDCl3) δ (ppm): 2.29 (s, 3H); 4.71 (s, 2H); 7.07-8.2 (m, aromatics, 8H). |
| 80% |
With triethylamine In toluene at 5 - 10℃; for 2h; |
1b Example 1b; Preparation of 3-Hydroxymethylphenyl Ester of the 2-Acetoxybenzoic Acid (Compound I-B) in Organic Solvent Immiscible With Water
3-hydroxymethylphenol (10 g, 0.08 moles) is dissolved in toluene (50 ml) containing triethylamine (9.8 g, 0.1 moles). To the so obtained solution an acetylsalicylic acid chloride solution (16 g, 0.08 moles) in toluene (50 ml) is added at a temperature of 5°-10° C. under stirring. The mixture is maintained at a temperature in the above mentioned range, under stirring for 2 hours, then poured in water and then extracted with dichloromethane (2×100 ml). The organic phase is separated, washed in sequence with a 25% w/v potassium carbonate solution, with water, with a 3% hydrochloric acid solution and lastly with water again, then anhydrified with sodium sulphate and the solvent evaporated under vacuum. The residue is crystallized from isopropanol. 3-hydroxymethylphenyl ester of the 2-acetoxybenzoic acid (45.8 g, 0.16 moles, yield 80%) is obtained. [00041] M.P.: 79°-80° C. 1H NMR(CDCl3) δ (pp): 2.29 (s, 3H); 4.71 (s, 2H); 7.07-8.2 (m, aromatics, 8H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






