Alternatived Products of [ 29559-24-8 ]
Product Details of [ 29559-24-8 ]
| CAS No. : | 29559-24-8 |
MDL No. : | MFCD26399832 |
| Formula : |
C7H6ClNO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
171.58
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 29559-24-8 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 29559-24-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 29559-24-8 ]
- 1
-
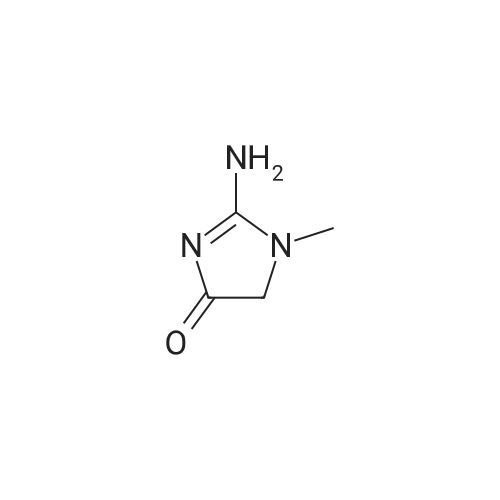 [ 60-27-5 ]
[ 60-27-5 ]

-
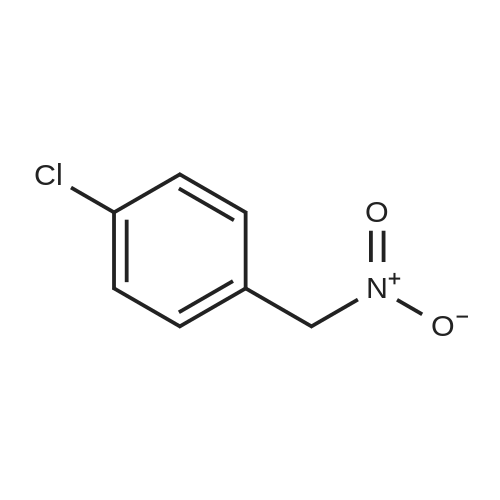 [ 29559-24-8 ]
[ 29559-24-8 ]

-
 [ 89114-31-8 ]
[ 89114-31-8 ]
- 2
-
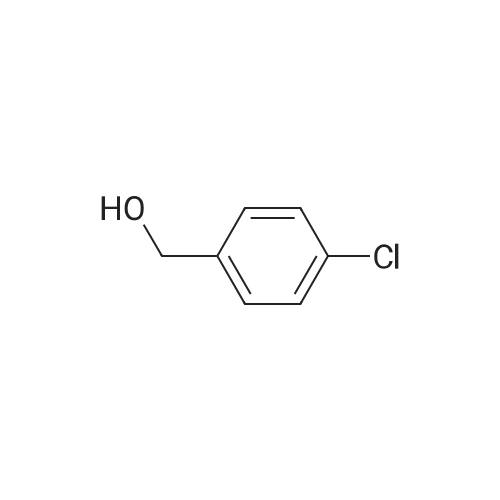 [ 873-76-7 ]
[ 873-76-7 ]

-
 [ 29559-24-8 ]
[ 29559-24-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 91.3% |
Stage #1: para-Chlorobenzyl alcohol With acetic acid; sodium nitrite In dichloromethane at 20℃; for 0.0833333h;
Stage #2: With hydrogenchloride In dichloromethane; water at 20℃; for 8h; |
2.1
(1) p-chlorobenzyl alcohol (28.5 g) was dissolved in dichloromethane, followed by sequential addition of sodium nitrite (27.6 g) and acetic acid (18.0 g). The reaction mixture was stirred at room temperature for 5 minutes, then concentrated hydrochloric acid was added and stirred at room temperature for 8 hours. After completion of the reaction, the reaction mixture was diluted with methylene chloride and filtered to remove sodium acetate. Dichloromethane, acetic acid and water were distilled off under reduced pressure to obtain 31.3 g of compound I. Compound I content of 95.5%, the yield of 91.3%. |
| 89 % Chromat. |
With hydrogenchloride; acetic acid; sodium nitrite In dichloromethane at 20℃; for 6h; |
|
- 3
-
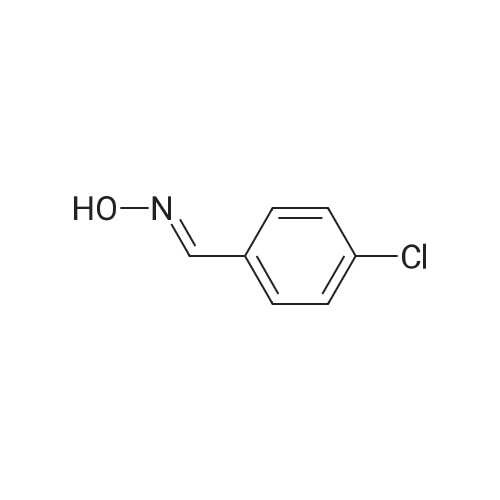 [ 3848-36-0 ]
[ 3848-36-0 ]

-
 [ 29559-24-8 ]
[ 29559-24-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86.74% |
With dihydrogen peroxide; sodium acetate; acetic acid at 100℃; for 6h; |
7 xample 7 Synthesis of p-chlorophenylnitromethane
a 250 mL four-necked flask of a thermometer and a stirrer, 23.5 g (0.15 mol) of p-chlorobenzaldehyde oxime was added.30% hydrogen peroxide 78g (over 300%),Acetic acid 30.4g,Sodium acetate 2.0 g was started to stir and heat.The reaction was carried out at 100 ° C for 6 hours.TLC monitored the reaction completely. After completion of the reaction, it was cooled to 20-25 ° C, and 150 ml of pure water was added thereto, followed by stirring for half an hour. After suction filtration, an orange solid was obtained, which was washed three times with 300 ml of purified water. P-chlorophenylnitromethane was obtained, dried and weighed to give a product of 19.8 g, yield 86.74%, HPLC: 97.9%. |
|
With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; |
|
|
With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 48h; |
|
Reference:
[1]Current Patent Assignee: INSTITUTE OF DAFENG MARINE INDUSTRY NANJING UNIV O; YANCHENG TEACHERS UNIVERSITY - CN108484408, 2018, A
Location in patent: Paragraph 0058-0074
[2]Vara, Brandon A.; Mayasundari, Anand; Tellis, John C.; Danneman, Michael W.; Arredondo, Vanessa; Davis, Tyler A.; Min, Jaeki; Finch, Kristin; Guy, R. Kiplin; Johnston, Jeffrey N.
[Journal of Organic Chemistry, 2014, vol. 79, # 15, p. 6913 - 6938]
[3]Tsukanov, Sergey V.; Johnson, Martin D.; May, Scott A.; Rosemeyer, Morgan; Watkins, Michael A.; Kolis, Stanley P.; Yates, Matthew H.; Johnston, Jeffrey N.
[Organic Process Research and Development, 2016, vol. 20, # 2, p. 215 - 226]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







