Alternatived Products of [ 306768-12-7 ]
Product Details of [ 306768-12-7 ]
| CAS No. : | 306768-12-7 |
MDL No. : | MFCD22419008 |
| Formula : |
C12H16INO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
333.17
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 306768-12-7 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 306768-12-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 306768-12-7 ]
- 1
-
 [ 24424-99-5 ]
[ 24424-99-5 ]

-
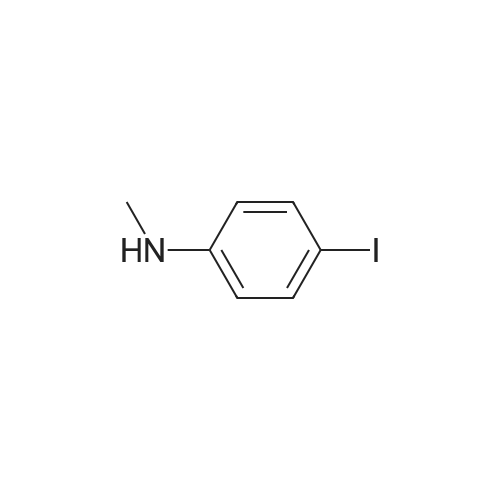 [ 60577-34-6 ]
[ 60577-34-6 ]

-
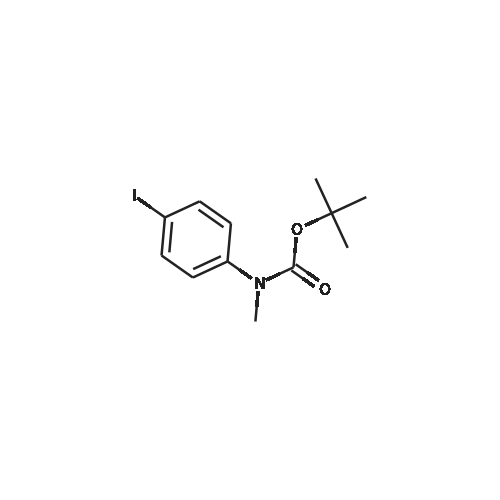 [ 306768-12-7 ]
[ 306768-12-7 ]
- 2
-
 [ 73183-34-3 ]
[ 73183-34-3 ]

-
 [ 306768-12-7 ]
[ 306768-12-7 ]

-
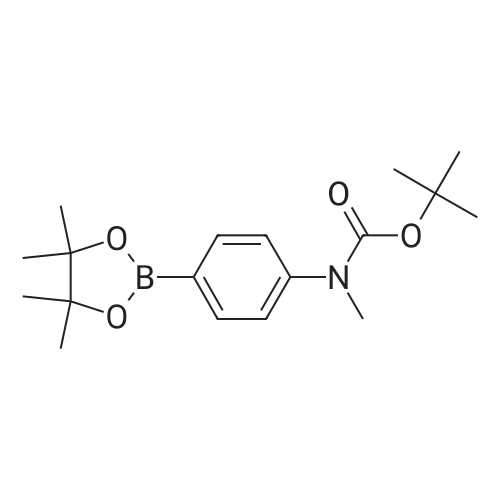 [ 916587-44-5 ]
[ 916587-44-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 63% |
With potassium acetate In dimethyl sulfoxide at 80℃; for 3.5h; |
|
| 63% |
With CH3COOK; PdCl2(Fe(C5H4P(C6H5)2)2)*CH2Cl2 In dimethyl sulfoxide (N2); addn. of soln. of carbamic acid deriv. in DMSO to mixture of boroncompd., palladium compd. and potassium acetate, stirring at 80°C for 3.5 h; cooling to room temp., addn. of ethyl acetate, washing with water, drying with MgSO4, concn., chromy. (silica gel, hexanes/ethyl acetate (8:1)),NMR; |
|
Reference:
[1]Lin, Chen; Drueckhammer, Dale G.
[New Journal of Chemistry, 2006, vol. 30, # 12, p. 1725 - 1730]
[2]Lin, Chen; Drueckhammer, Dale G.
[New Journal of Chemistry, 2006, vol. 30, # 12, p. 1725 - 1730]
- 3
-
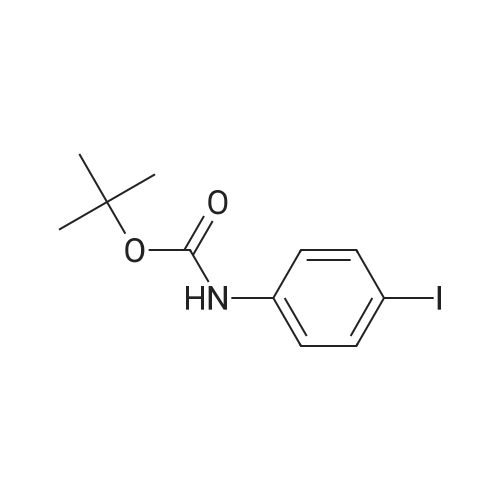 [ 159217-89-7 ]
[ 159217-89-7 ]

-
 [ 74-88-4 ]
[ 74-88-4 ]

-
 [ 306768-12-7 ]
[ 306768-12-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
With sodium hydride In tetrahydrofuran at 0 - 20℃; for 6h; |
|
| 75% |
Stage #1: N-Boc-4-iodoaniline With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h;
Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 0 - 25℃; for 2h; |
1 Step 1 - Tert-butyl N-[4-[1-(4-chlorophenyl)-7-isopropoxy-6-methoxy-3-oxo-1,4-dihydroisoquinolin-2-yl]phenyl]-N-methylcarbamate
To a solution of tert-butyl N-(4-iodophenyl)carbamate (1.00 g, 3.13 mmol, CASNo. 159217-89-7) in DMF (10 mL) was added NaH (376 mg, 9.40 mmol, 60% dispersion in mineral oil) at 0 °C stirred for 30 mins, and CH3I (2.22 g, 15.7 mmol) was added. Then the mixture was stirred at 25 °C for 2 hours. On completion, the reaction mixture was poured into the H2O (10 mL), then extracted with EtOAc (6.0 mL x 3). The organic layer was separated, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the residue. The residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl acetate = 10/1 to 2/3) to give the title compound (840 mg, 75% yield) as a white solid. LC-MS (ESI+) m/z 278.2 (M+H-56)+. |
| 75% |
Stage #1: N-Boc-4-iodoaniline With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h;
Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 0 - 25℃; for 2h; |
1 Step 1 - Tert-butyl N-[4-[1-(4-chlorophenyl)-7-isopropoxy-6-methoxy-3-oxo-1,4-dihydroisoquinolin-2-yl]phenyl]-N-methylcarbamate
To a solution of tert-butyl N-(4-iodophenyl)carbamate (1.00 g, 3.13 mmol, CASNo. 159217-89-7) in DMF (10 mL) was added NaH (376 mg, 9.40 mmol, 60% dispersion in mineral oil) at 0 °C stirred for 30 mins, and CH3I (2.22 g, 15.7 mmol) was added. Then the mixture was stirred at 25 °C for 2 hours. On completion, the reaction mixture was poured into the H2O (10 mL), then extracted with EtOAc (6.0 mL x 3). The organic layer was separated, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the residue. The residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl acetate = 10/1 to 2/3) to give the title compound (840 mg, 75% yield) as a white solid. LC-MS (ESI+) m/z 278.2 (M+H-56)+. |
Reference:
[1]Cho, Sung Jin; Elbatrawy, Ahmed A.; Han, Ye Ri; Jeon, Hui-Jeon; Jeon, Yong Hyun; Kang, Kyung-Ku; Kim, Dong-Su; Kim, Kil Soo; Lee, Da Sol; Lee, Sang Bong; Lee, Sijoon; Nam, Ghilsoo; Sung, Soo-Eun
[Journal of Materials Chemistry B, 2021, vol. 9, # 24, p. 4857 - 4862]
[2]Current Patent Assignee: KYMERA THERAPEUTICS INC - WO2021/188948, 2021, A1
Location in patent: Paragraph 001860; 001861-001862
[3]Current Patent Assignee: KYMERA THERAPEUTICS INC - WO2021/188948, 2021, A1
Location in patent: Paragraph 001860; 001861-001862
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








