Alternatived Products of [ 348-84-5 ]
Product Details of [ 348-84-5 ]
| CAS No. : | 348-84-5 |
MDL No. : | MFCD04973768 |
| Formula : |
C9H8ClF3O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
224.61
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 348-84-5 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 348-84-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 348-84-5 ]
- 1
-
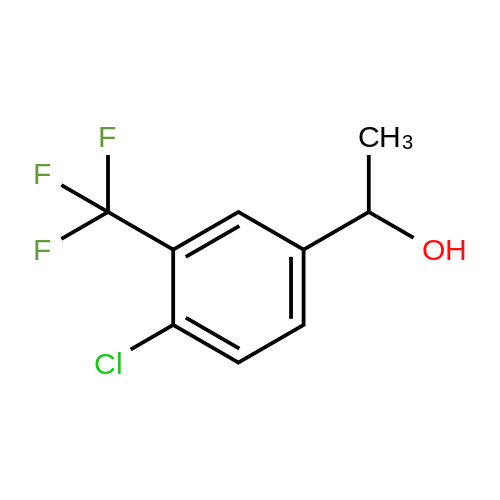 [ 348-84-5 ]
[ 348-84-5 ]

-
 [ 2366-80-5 ]
[ 2366-80-5 ]
- 2
-
4-chloro-3-trifluoromethyl-phenyl magnesium bromide
[ No CAS ]

-
 [ 348-84-5 ]
[ 348-84-5 ]
- 3
-
 [ 348-84-5 ]
[ 348-84-5 ]

-
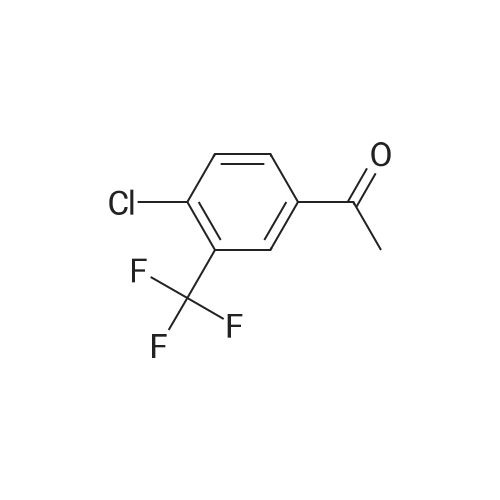 [ 129825-11-2 ]
[ 129825-11-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 95% |
With oxalyl dichloride; triethylamine; In dichloromethane; dimethyl sulfoxide; at -78℃; |
[4-CHLORO-3- (TRIFLUOROMETHYL)] benzylaldhyde (3.0 g, 14.4 mmol) in diethyl ether was cooled [TO-78 C. METHYLMAGNESIUM] chloride (3. 0M solution in THF, 29 mmol) was added dropwise. The reaction was warmed up slowly [TO-20] C. TLC showed the reaction was complete. It was quenched with saturated amonium chloride solution. The aqueous layer was extracted with ethyl acetate, organic layer wre combined and washed with water, brine, and dried with magnesium sulfate. This gave [1- (4-CHLORO-3-TRIFLUOROMETHYLPHENYL)] ethanol (3.2g, [99%)] after the solvent was removed under reduced pressure. [1- (4-CHLORO-4-TRIFLUOROMETHYL-] phenyl) ethanol was then oxidized by [SWERN] oxidation (2.5 eq. DMSO, 1.2 eq. oxyal chloride, 5 eq. triethylamine, [60ML] of [DICHLOROMETHANE,-78] C). The resulting 1- (3-chloro-4- trifluoromethylphenyl) ethanone (3 g, 95% yield) was brominated according to a literature [PROCEDURE (HORNG-CHIH, HUANG ET AL. , J. MED. CHEM. ; 1996, VOL. 39,253-266). 4-CHLORO-3- (TRIFLUOROMETHYL) -PHENACYL BROMIDE WAS OBTAINED IN 70% YIELD. LC/MS (ES+) M/E 303 [M+1]] |
| 87% |
With pyridinium chlorochromate; In dichloromethane; at 20℃; |
The compound 149A (2.62 g, 11.7 mmol) is dissolved in53 mL of DCM in the presence of 3.8 g of celite. PCC(3.77 g, 17.5 mmol) is added at room temperature and the reaction medium is stirred overnight before being filtered. The filtrate is concentrated under reduced pressure. The thereby obtained residue is purified on a column of 80 g of silica (32 mL / min, gradient of 0% to 30% EtOAc in heptane in 26 minutes), in order to obtain the compound 149B (2.27 g, 87%) .HPLC: RT = 5.84 min, 97% (colonne XBridge)1H NMR, dmso-de, delta (ppm) : 2.65 (s, 3H); 7.92 (d, IH); 8.22-8.27 (m, 2H) . |
- 4
-
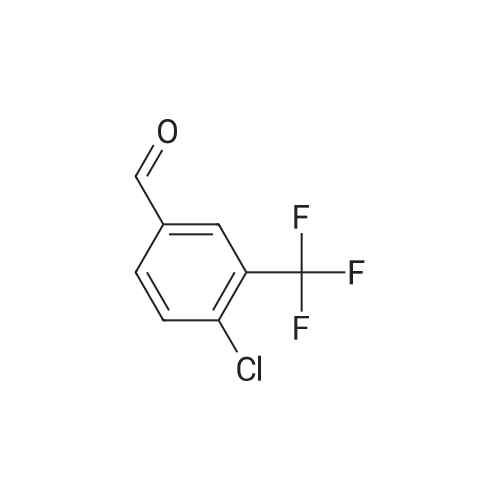 [ 34328-46-6 ]
[ 34328-46-6 ]

-
 [ 348-84-5 ]
[ 348-84-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
With methylmagnesium chloride; In tetrahydrofuran; diethyl ether; at -78 - -20℃; |
[4-CHLORO-3- (TRIFLUOROMETHYL)] benzylaldhyde (3.0 g, 14.4 mmol) in diethyl ether was cooled [TO-78 C. METHYLMAGNESIUM] chloride (3. 0M solution in THF, 29 mmol) was added dropwise. The reaction was warmed up slowly [TO-20] C. TLC showed the reaction was complete. It was quenched with saturated amonium chloride solution. The aqueous layer was extracted with ethyl acetate, organic layer wre combined and washed with water, brine, and dried with magnesium sulfate. This gave [1- (4-CHLORO-3-TRIFLUOROMETHYLPHENYL)] ethanol (3.2g, [99%)] after the solvent was removed under reduced pressure. [1- (4-CHLORO-4-TRIFLUOROMETHYL-] phenyl) ethanol was then oxidized by [SWERN] oxidation (2.5 eq. DMSO, 1.2 eq. oxyal chloride, 5 eq. triethylamine, [60ML] of [DICHLOROMETHANE,-78] C). The resulting 1- (3-chloro-4- trifluoromethylphenyl) ethanone (3 g, 95% yield) was brominated according to a literature [PROCEDURE (HORNG-CHIH, HUANG ET AL. , J. MED. CHEM. ; 1996, VOL. 39,253-266). 4-CHLORO-3- (TRIFLUOROMETHYL) -PHENACYL BROMIDE WAS OBTAINED IN 70% YIELD. LC/MS (ES+) M/E 303 [M+1]] |
- 5
-
 [ 34328-46-6 ]
[ 34328-46-6 ]

-
 [ 75-16-1 ]
[ 75-16-1 ]

-
 [ 348-84-5 ]
[ 348-84-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 62% |
|
<strong>[34328-46-6]4-chloro-3-trifluoromethyl-benzaldehyde</strong> (6.19 g, 29.7 mmol) is dissolved in 124 mL of THF under a nitrogen atmosphere. The reaction medium is cooled to -78C before adding dropwise MeMgBr (13 mL, 3M / Et2O, 38.6 mmol) , and then stirred for a further 2 hours at this low temperature, and finally neutralized by adding 60 mL of a saturated NH4Cl solution. The reaction medium is extracted twice with ethyl acetate. The organic phases are combined, washed with a saturated NaCl solution, and then dried on magnesium sulfate, filtered and concentrated under reduced pressure. The thereby obtained residue is purified on a column of 120 g of silica (92 mL / min; gradient of 0% to 35% ethyl acetate in heptane in 40 min), in order to obtain the compound 149A (5.71 g, 62%) .HPLC: RT = 5.71 min, 98% (colonne XBridge)1H NMR, dmso-de, delta (ppm) : 1.33 (d, 3H); 4.81 (quintet, IH); 5.46 (d, IH, exch) ; 7.60-7.69 (m, 2H); 7.81 (s, IH) . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







