| 90% |
With potassium permanganate; water; In water; at 25℃; for 6h; |
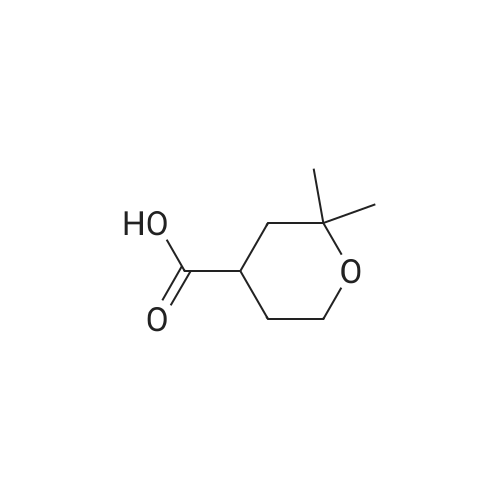
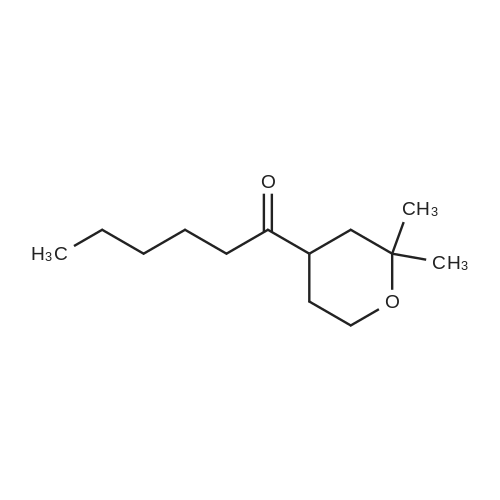
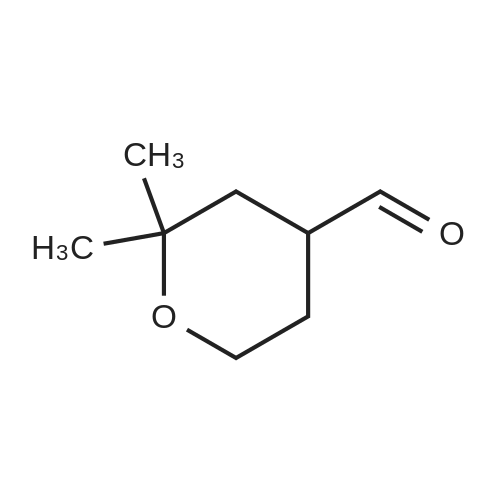
IO a solution ot mercury sultate (LA g) and SULTUNC acid (5"/0, 6UU ML), was gradually added dimethylvinylethynylcarbinole (230 g) with vigerous stirring FOR-1 h and the temperature was raised slowly to 85 C. Mercury sulfate (18 g) was added in portions over 4 h at 85 C. The upper layer was separated, and the lower layer was neutralized with potassium carbonate and extracted with ether twice. The separated upper layer was added to the ethereal extracts, washed with potassium carbonate, water and dried over magnesium sulfate. The ether was evaporated, and the residue distilled under vacuum, then distilled at 1 atm (168-175 C) to provide the product pyrone (140 g, 53%). A suspension of sodium (11 g) in dry toluene (150 mL) was heated to reflux, stirred vigerously, cooled to room temperature, and a first part of solution (5-6 mL) of chloroacetic acid ethyl ester (63 g, 0.5 mol) and the above 2, 2-DIMETHYLTETRAHYDROPYRANE-4-ONE (64 g, 0.5 mol) was added. The remaining part of the solution was added dropwise at 23 C. After 3-4 h and full consumption of sodium, dry methanol (8 mL) was added. The mixture was carefully quenched into ice water (100 mL), ether was added, the upper layer separated, the aqueous extracted with ether, the ethereal extracts combined, washed with water, and dried over magnesium sulfate. The solvent was evaporated, and the residue was distilled in vacuo (101 C, 2 mmHg) to provide the product (74 g, 70%). This product 5, 5-dimethyl-1, 6-dioxaspyro-2,5-octane-2-carbonic acid ethyl ester (214 g, 1 mol) was added to a solution of sodium hydroxide (40 g, 1 mol) in water (100 mL) with stirring over 30 min at 30 C. The reaction mixture was stirred for 2 h, water (200 ML) was added to dissolve precipitates, and the aqueous solution of the sodium salt of 5, 5-dimethyl-1, 6- dioxaspyro-2,5-octane-2-carbonic acid was heated to 90 C. Hydrochloric acid (1 mol, 15-20%) was added dropwise for 4 h, the forming aldehyde was simultaneously distilled, saturated with NACI, extracted with ether (3X200 mL), the organic extracts dried over anhydrous magnesium sulfate, and concentrated in vacuo. The residue was distilled in vacuo (61 C at 7 MMHG) to provide the aldehyde intermediate (80 g, 55%). To a cold 2,2-dimethyl-4-formyltetrahydropyran (17 g, 0.5 mol) was added a solution of potassium permanganate (79 g, 0.5 mol) in water (1 L), keeping the temperature at 25 C. The mixture was maintained for 6 h, filtered from MNO2, washed with water, the combined filtrate evaporated to the volume of 175 ML, acidified with HC1, extracted with diethyl ether, and the organic extracts dried over anhydrous magnesium sulfate and concentrated in vacuo. The residue was distilled under vacuum at 123 C (5 MMHG) to yield 71 g (90%) of the desired acid. This carboxylic acid was incorporated into Example 82 by following the procedures of Examples 78 and 71, US Patent 6,489, 354 and Example 11 from tent-butyl 3-BENZYL-1-OXA-8- azaspiro [4.5] decane-8-carboxylate. Mass Spectrum m/e = 585 (M+1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping