Alternatived Products of [ 4146-23-0 ]
Product Details of [ 4146-23-0 ]
| CAS No. : | 4146-23-0 |
MDL No. : | MFCD00956161 |
| Formula : |
C8H6ClNS
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
183.66
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 4146-23-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 4146-23-0 ]
- 1
-
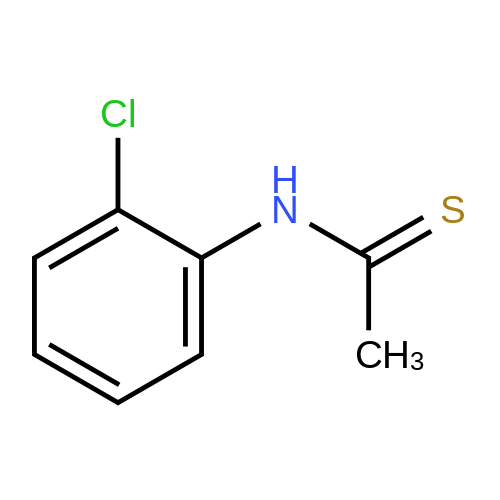 [ 39184-83-3 ]
[ 39184-83-3 ]

-
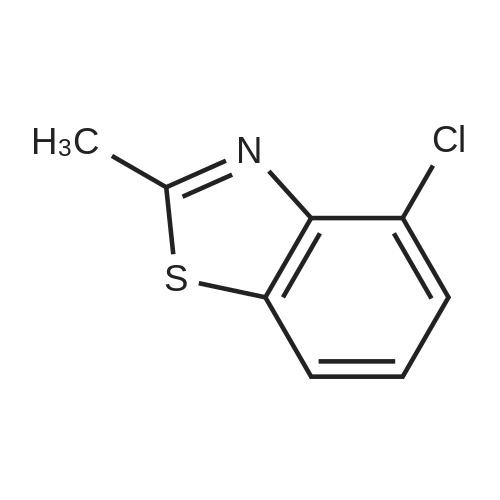 [ 4146-23-0 ]
[ 4146-23-0 ]
- 2
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 854084-78-9 ]
[ 854084-78-9 ]
- 3
-
 [ 113570-95-9 ]
[ 113570-95-9 ]

-
 [ 4146-23-0 ]
[ 4146-23-0 ]
- 4
-
 [ 88142-15-8 ]
[ 88142-15-8 ]

-
 [ 4146-23-0 ]
[ 4146-23-0 ]
- 5
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 101251-76-7 ]
[ 101251-76-7 ]
- 6
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 89978-25-6 ]
[ 89978-25-6 ]
- 7
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
4,6,7-trichloro-2-methyl-benzothiazole
[ No CAS ]
- 8
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
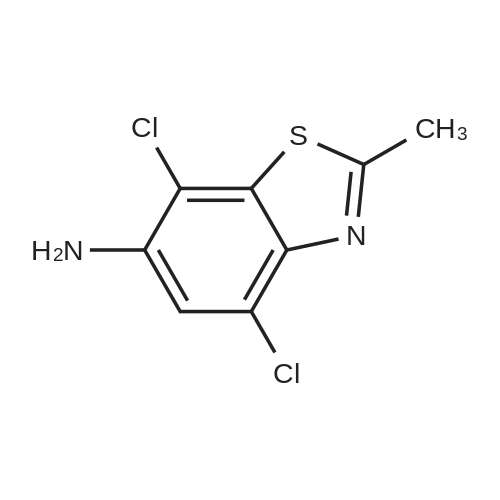 [ 21325-10-0 ]
[ 21325-10-0 ]
- 9
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 854059-22-6 ]
[ 854059-22-6 ]
- 10
-
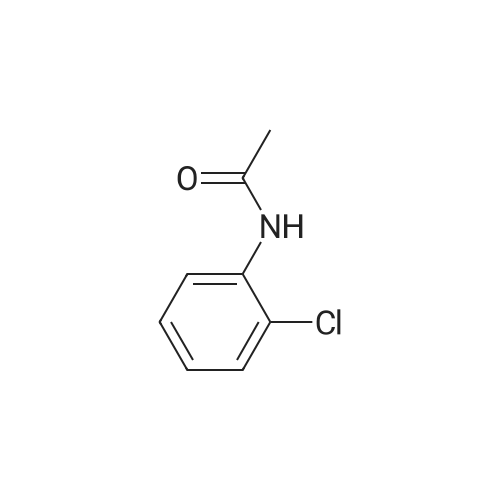 [ 533-17-5 ]
[ 533-17-5 ]

-
 [ 4146-23-0 ]
[ 4146-23-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
The following compounds of formula I are those which are preferred within the scope of the invention: ... 2-t-butyl-5-methoxy-6-isothiocyanatobenzothiazole, 5-amino-2-methylbenzothiazole, 2-(2-dimethylaminoethenyl)-benzothiazole, 2-propionylbenzothiazole, 4-chloro-2-methylbenzothiazole, 5,6-dimethoxy-2-methylbenzothiazole, 2-methylnaphtho[1,2-d]thiazole, 6-methoxy-2-methylbenzothiazole, ... |
- 12
-
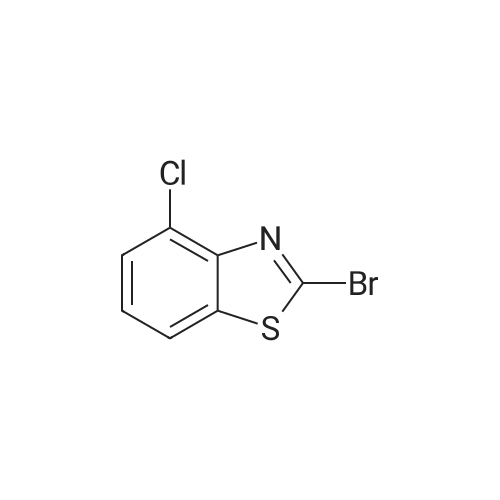 [ 3622-40-0 ]
[ 3622-40-0 ]

-
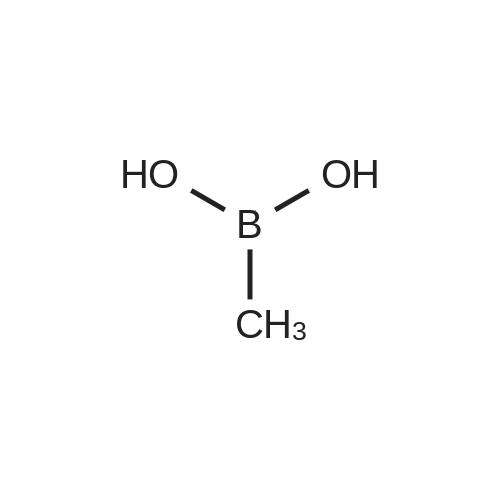 [ 13061-96-6 ]
[ 13061-96-6 ]

-
 [ 4146-23-0 ]
[ 4146-23-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 96% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In ethanol; water; toluene; at 80℃; for 18.0h;Inert atmosphere; Schlenk technique; |
2-bromo-4-chlorobenzo [d] thiazole (1.99 g, 8 mmol) was added to a 250 mL Schlenk flask which was well-methylboronic acid (484 mg, 8.08 mmol), 2M Na2CO3 aqueous solution (10 mL, 20 mmol), anhydrous ethanol (20 mL)Anhydrous toluene (20 mL) and Pd (PPh3) 4 (279 mg, 0.24 mmol) were added. After degassing, it was substituted with Ar and stirred vigorously at 80 C for 18 hours.After a certain period of time, the mixture was cooled to room temperature and the organic layer was separated. The water layer was washed with dichloromethane (20 mL x 2).The organic layer was collected, passed through a silica gel pad, and concentrated.The crude product was purified by column chromatography (n-hexane / ethyl acetate = 100/1) to obtain 4-chloro-2-methylbenzo [d] thiazole. Yield 1.41 g (96%). |
| 96% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In ethanol; water; toluene; for 18.0h;Reflux; Schlenk technique; |
Well dried and Ar-substituted 250 mL Schlenk flask2-bromo-4-chlorobenzo [d] thiazole (1.99 g, 8 mmol), onic (484 mg, 8.08 mmol), 2M Na2CO3 aqueous solution (10 mL, 20 mmol),Anhydrous ethanol (20 mL),Anhydrous toluene (20 mL),Pd (PPh3) 4 (279 mg, 0.24 mmol) was added thereto.After degassing, it was substituted with Ar and stirred vigorously at 80 C for 18 hours.After a certain period of time, the mixture was cooled to room temperature and the organic layer was separated.The water layer was washed with dichloromethane (20 mL x 2).The organic layer was collected, passed through a silica gel pad, and concentrated.Crude product was purified by column chromatography(n-hexane / ethyl acetate = 100/1)To give 4-chloro-2-methylbenzo [d] thiazole. Yield 1.41 g (96%). |
- 13
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 87-62-7 ]
[ 87-62-7 ]

-
C16H16N2S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 83% |
With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); In 1,4-dioxane; for 18.0h;Inert atmosphere; Schlenk technique; Reflux; |
100 mL Schlenk flask well dried and replaced with ArChloro-2-methylbenzo [d] thiazole (551 mg, 3 mmol) prepared in the above step (a)Pd2 (dba) 3 (165 mg, 0.18 mmol), DPPF (200 mg, 0.36 mmol), sodium tert-butoxide (432 mg, 4.5 mmol)Anhydrous 1,4-dioxane (15 mL) and 2,6-dimethylaniline (406 muL, 3.3 mmol) were added and refluxed for 18 hours. After a certain period of time, the reaction mixture was cooled to room temperature and the reaction was terminated by adding an aqueous sat'd NH4Cl solution (15 mL).The organic layer was separated, passed through a silica gel pad, and concentrated. Crude product was recrystallized under ethanol to obtain Compound 1-4. Yield 668 mg (83%). |
- 14
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 87-62-7 ]
[ 87-62-7 ]

-
C37H36HfN2S
[ No CAS ]
- 15
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
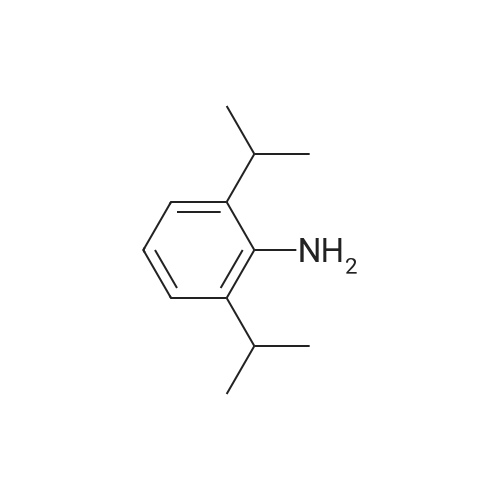 [ 24544-04-5 ]
[ 24544-04-5 ]

-
C20H24N2S
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 92% |
With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); In 1,4-dioxane; for 18.0h;Inert atmosphere; Schlenk technique; Reflux; |
100 mL Schlenk flask well dried and replaced with ArA 4-chloro-2-methyl-benzo [d] thiazole (551 mg, 3 mmol) prepared in Step (a) in Example 5,Pd2 (dba) 3 (165 mg, 0.18 mmol), DPPF (200 mg, 0.36 mmol), sodium tert-butoxide (432 mg, 4.5 mmol)Anhydrous 1,4-dioxane (15 mL) and 2,6-diisopropylaniline (622 muL, 3.3 mmol) were added and refluxed for 18 hours.After a certain period of time, the reaction mixture was cooled to room temperature and the reaction was terminated by adding an aqueous sat'd NH4Cl solution (15 mL).The organic layer was separated, passed through a silica gel pad, and concentrated. Crude product was recrystallized under ethanol to obtain compound 1-10. Yield 896 mg (92%). |
| 92% |
With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); sodium carbonate; In 1,4-dioxane; for 18.0h;Reflux; Schlenk technique; |
A 100 mL Schlenk flask, well dried and replaced with Ar, was added to the solution prepared in step (a) of Example 54-chloro-2-methylbenzo [d] thiazole(551 mg, 3 mmol), Pd2 (dba) 3(165 mg, 0.18 mmol),DPPF (200 mg, 0.36 mmol),sodium tertbutoxide(432 mg, 4.5 mmol),Anhydrous 1,4-dioxane (15 mL), 2,6-diisopropylaniline (622 muL, 3.3 mmol) was added and refluxed for 18 hours.After a certain period of time, the reaction mixture was cooled to room temperature and the reaction was terminated by adding an aqueous sat'd NH4Cl solution (15 mL).The organic layer was separated, passed through a silica gel pad, and concentrated. Crude product was recrystallized under ethanol to obtain compound 1-10. Yield 896 mg (92%). |
- 16
-
 [ 4146-23-0 ]
[ 4146-23-0 ]

-
 [ 24544-04-5 ]
[ 24544-04-5 ]

-
C41H44HfN2S
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping












