Alternatived Products of [ 41855-35-0 ]
Product Details of [ 41855-35-0 ]
| CAS No. : | 41855-35-0 |
MDL No. : | MFCD00188456 |
| Formula : |
C18H26O7
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
354.39
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 41855-35-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 41855-35-0 ]
- 1
-
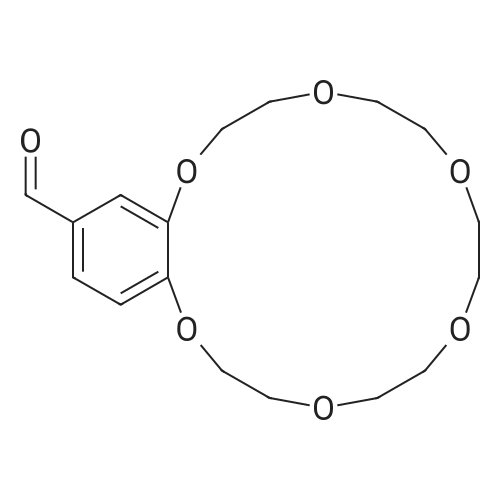 [ 60835-74-7 ]
[ 60835-74-7 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 135442-24-9 ]
[ 135442-24-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 15% |
With potassium hydroxide In methanol at 50 - 60℃; for 240h; |
|
- 2
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 41757-97-5 ]
[ 41757-97-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 92% |
With sodium tetrahydroborate In ethanol for 18h; Ambient temperature; |
|
- 3
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
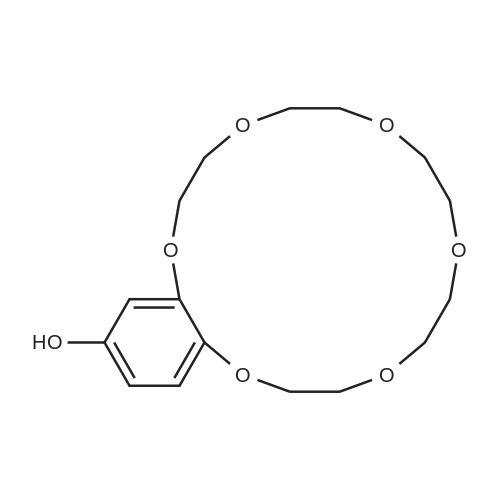 [ 76427-68-4 ]
[ 76427-68-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With peracetic acid; sodium hydroxide Multistep reaction; |
|
|
Multi-step reaction with 2 steps
1.1: toluene-4-sulfonic acid; 3-chloro-benzenecarboperoxoic acid / dichloromethane / 20 °C / Inert atmosphere
2.1: sodium hydroxide / water / 3 h / 50 °C
2.2: pH 2 |
|
Reference:
[1]Kikukawa, Kiyoshi; He, Gong-Xin; Abe, Akito; Goto, Tokio; Arata, Ryozo; et al.
[Journal of the Chemical Society. Perkin transactions II, 1987, p. 135 - 142]
[2]Paramonov, Sergey V.; Lokshin, Vladimir; Smolentsev, Artem B.; Glebov, Evgeni M.; Korolev, Valeri V.; Basok, Stepan S.; Lysenko, Konstantin A.; Delbaere, Stéphanie; Fedorova, Olga A.
[Tetrahedron, 2012, vol. 68, # 38, p. 7873 - 7883]
- 4
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 81117-71-7 ]
[ 81117-71-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 43% |
With hydrogenchloride; amalgamated zinc In 1,4-dioxane; water; toluene for 40h; Heating; |
|
Reference:
[1]Shkinev, A. V.; Asrarov, M. I.; Gagel'gans, A. I.; Saifullina, N. Zh.; Mukhamedzhanova, E. A.; Tashmukhamedova, A. K.
[Chemistry of Natural Compounds, 1983, vol. 19, # 5, p. 597 - 600][Khimiya Prirodnykh Soedinenii, 1983, # 5, p. 634 - 637]
- 5
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 77586-36-8 ]
[ 77586-36-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 74% |
With bromine In chloroform for 2h; |
|
- 6
-
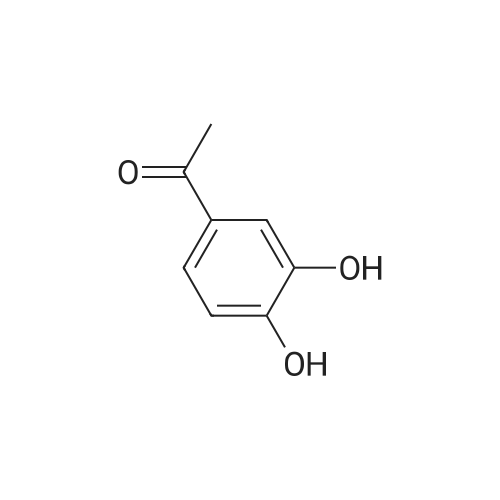 [ 1197-09-7 ]
[ 1197-09-7 ]

-
 [ 76871-59-5 ]
[ 76871-59-5 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium carbonate In acetone at 50 - 60℃; for 65h; Yield given; |
|
- 7
-
 [ 1197-09-7 ]
[ 1197-09-7 ]

-
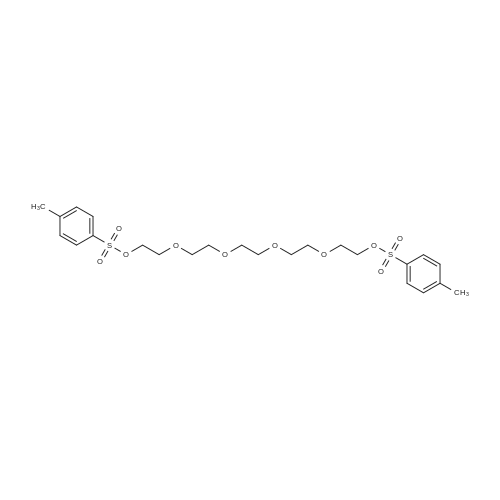 [ 41024-91-3 ]
[ 41024-91-3 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 48% |
With sodium hydroxide In water; butan-1-ol for 72h; Heating; |
|
- 8
-
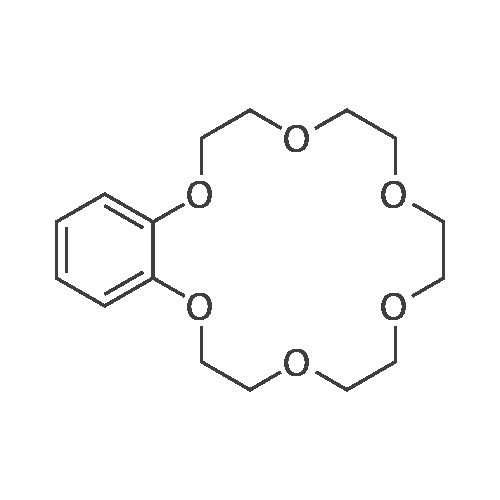 [ 14098-24-9 ]
[ 14098-24-9 ]

-
 [ 108-24-7 ]
[ 108-24-7 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]
- 9
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 90-02-8 ]
[ 90-02-8 ]

-
 [ 105-58-8 ]
[ 105-58-8 ]

-
 [ 154615-37-9 ]
[ 154615-37-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With piperidine; potassium <i>tert</i>-butylate In ethanol Heating; |
|
- 10
-
 [ 14098-24-9 ]
[ 14098-24-9 ]

-
 [ 64-19-7 ]
[ 64-19-7 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 80% |
With methanesulfonic acid; phosphorus pentoxide; at 20℃; for 6h; |
General procedure: A modification of the previously reported procedure23 was used. Commercially available Eaton's reagent (ca. 8 ml that corresponds to 6.28 mmol of phosphorus(V) oxide) and acetic acid (0.20 ml, 3.45 mmol) were mixed at room temperature. Then naphtho-15-crown-5 ether (1 g, 3.14 mmol) was added. The reaction mixture was stirred at room temperature for ca. 6 h and then poured into water. The suspension was extracted with dichloromethane and the combined organic phases were washed with water. Removal of the dried (MgSO4) solvent gave a dark brown oil, which upon repeated extractions with hot heptane, afforded the product as an off-white solid (1.02 g, 90%), mp 126-129 C. |
Reference:
[1]Chemistry of Natural Compounds,1983,vol. 19,p. 597 - 600
Khimiya Prirodnykh Soedinenii,1983,p. 634 - 637
[2]Tetrahedron,2012,vol. 68,p. 7873 - 7883
[3]Bulletin of the Chemical Society of Japan,1980,vol. 53,p. 2304 - 2308
- 11
-
 [ CAS Unavailable ]
[ CAS Unavailable ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 87% |
With water In chloroform for 1h; |
|
- 12
-
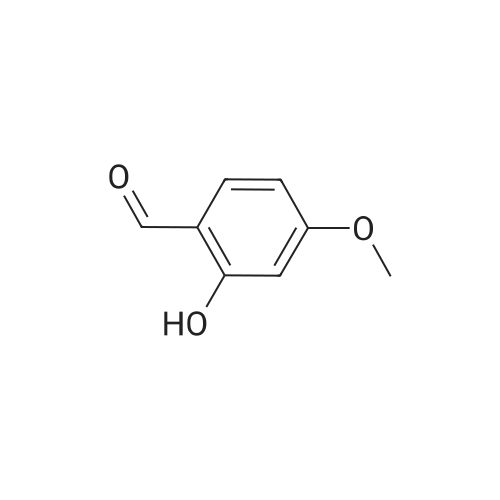 [ 673-22-3 ]
[ 673-22-3 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 105-58-8 ]
[ 105-58-8 ]

-
 [ 154615-38-0 ]
[ 154615-38-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With piperidine; potassium <i>tert</i>-butylate In ethanol Heating; |
|
- 13
-
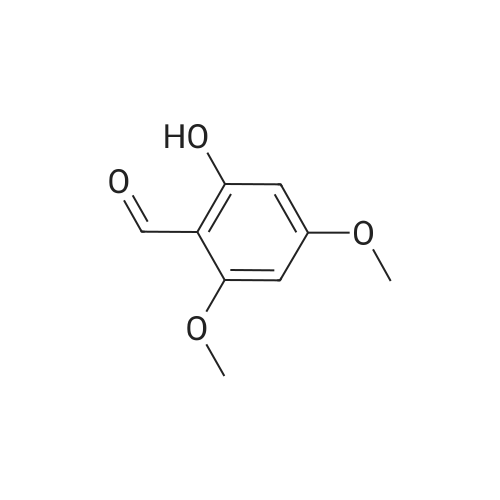 [ 708-76-9 ]
[ 708-76-9 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 105-58-8 ]
[ 105-58-8 ]

-
 [ 154615-39-1 ]
[ 154615-39-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With piperidine; potassium <i>tert</i>-butylate In ethanol Heating; |
|
- 14
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
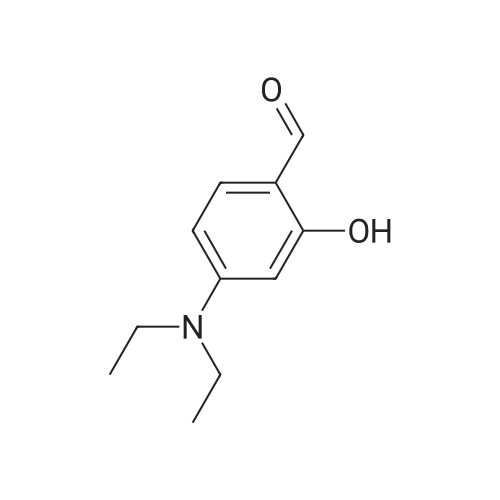 [ 17754-90-4 ]
[ 17754-90-4 ]

-
 [ 105-58-8 ]
[ 105-58-8 ]

-
 [ 154615-40-4 ]
[ 154615-40-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With piperidine; potassium <i>tert</i>-butylate In ethanol Heating; |
|
- 15
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 76427-66-2 ]
[ 76427-66-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
With toluene-4-sulfonic acid; 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; Inert atmosphere; |
4.3. Typical procedure for Bayer-Villiger oxidation
General procedure: An adaptation of the previously reported procedures [9] and [10a] was used. m-Chloroperbenzoic acid (70%, 3 mmol) was added to a stirred solution of ketone 10 (1.5 mmol), TsOH (1 mmol) in dichloromethane (10 ml) in a single portion. The mixture was stirred at room temperature overnight under argon atmosphere, then filtered and the precipitate formed was washed with dichloromethane. The organic phase was washed with 10% aqueous sodium bisulfite solution (2×20 ml), a saturated aqueous NaHCO3 solution (3×20 ml), brine (2×20 ml) then dried (MgSO4) and evaporated. |
| 65% |
With peracetic acid In acetic acid at 30 - 32℃; for 5h; |
|
Reference:
[1]Location in patent: experimental part
Paramonov, Sergey V.; Lokshin, Vladimir; Smolentsev, Artem B.; Glebov, Evgeni M.; Korolev, Valeri V.; Basok, Stepan S.; Lysenko, Konstantin A.; Delbaere, Stéphanie; Fedorova, Olga A.
[Tetrahedron, 2012, vol. 68, # 38, p. 7873 - 7883]
[2]Wada, Fumio; Arata, Ryozo; Goto, Tokio; Kikukawa, Kiyoshi; Matsuda, Tsutomu
[Bulletin of the Chemical Society of Japan, 1980, vol. 53, # 7, p. 2061 - 2063]
- 16
-
 [ 41757-97-5 ]
[ 41757-97-5 ]

-
 [ 41855-35-0 ]
[ 41855-35-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With chromium(VI) oxide; sulfuric acid In acetone Yield given; |
|
- 17
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 160951-74-6 ]
[ 160951-74-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrazine hydrate In ethanol for 24h; Heating; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
aus 3,4-Dihydroxy-acetophenon, CsF, entspr. Polyethylenglykolditosylat; |
|
- 19
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
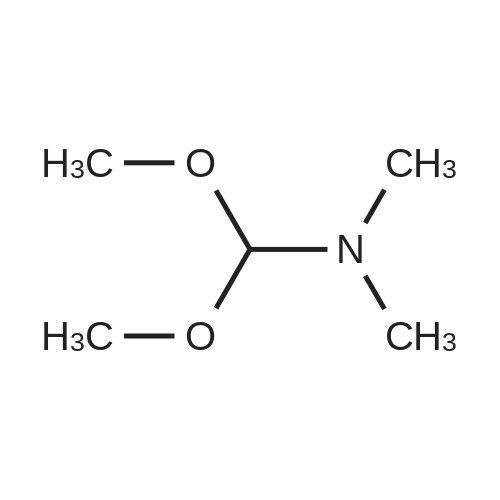 [ 4637-24-5 ]
[ 4637-24-5 ]

-
 [ 330445-31-3 ]
[ 330445-31-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
4'-acetylbenzo-18-crown-6; <i>N</i>,<i>N</i>-dimethyl-formamide dimethyl acetal
Stage #2: With hydrazine In ethanol |
|
- 20
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 75382-99-9 ]
[ 75382-99-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 21
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 106875-49-4 ]
[ 106875-49-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 22
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 75383-10-7 ]
[ 75383-10-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 23
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 106888-32-8 ]
[ 106888-32-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 24
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 75383-06-1 ]
[ 75383-06-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 25
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 75400-94-1 ]
[ 75400-94-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 26
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 75383-07-2 ]
[ 75383-07-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|
- 27
-
 [ 41855-35-0 ]
[ 41855-35-0 ]

-
 [ 75383-08-3 ]
[ 75383-08-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 1.) CH3COOOH; 2.) aq. NaOH
2: NaOH |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping















