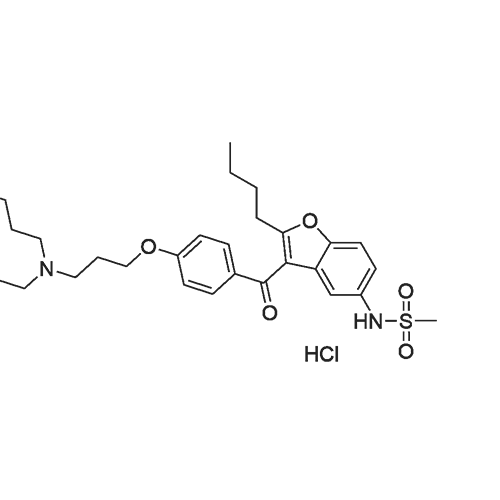
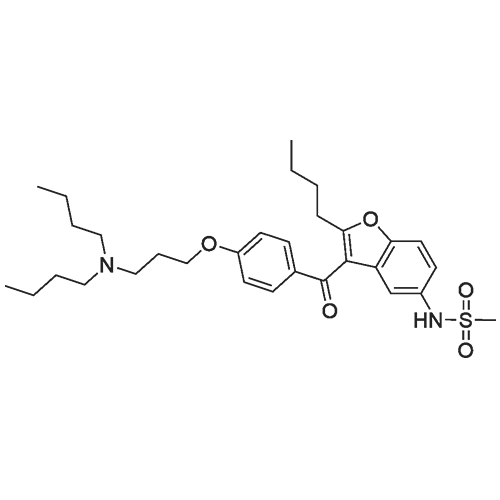
|
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 3.5 h / 10 - 20 °C
2: iron(III) chloride / dichloromethane / 5 - 40 °C
3: sodium hydroxide / methanol / 2 h / 65 °C |
|
|
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 6 h / 10 - 20 °C
2: aluminum (III) chloride / dichloromethane / 5 - 40 °C
3: hydrogenchloride; water / methanol / 3 h / 55 - 60 °C |
|
|
Multi-step reaction with 3 steps
1.1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
1.2: 0.83 h / 40 - 45 °C
2.1: sodium hydroxide / methanol / 3 h / Reflux
2.2: pH 6
3.1: pyridine / butan-1-ol / 14 h / 70 °C |
|
|
Multi-step reaction with 3 steps
1.1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
1.2: 0.83 h / 40 - 45 °C
2.1: sodium hydroxide / methanol / 3 h / Reflux
2.2: pH 6
3.1: pyridine / butan-1-ol / 14 h / 70 °C |
|
|
Multi-step reaction with 3 steps
1.1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
1.2: 0.83 h / 40 - 45 °C
2.1: sodium hydroxide / methanol / 3 h / Reflux
2.2: pH 6
3.1: sodium iodide; pyridine / butanone / 16.25 h / Reflux |
|
|
Multi-step reaction with 3 steps
1.1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
1.2: 0.83 h / 40 - 45 °C
2.1: sodium hydroxide; water / methanol / 3 h / pH 6 / Reflux
3.1: triacetoxyborohydride / dichloromethane / 12 h / 20 °C |
|
|
Multi-step reaction with 3 steps
1.1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
1.2: 0.83 h / 40 - 45 °C
2.1: sodium hydroxide; water / methanol / 3 h / pH 6 / Reflux
3.1: sodium tetrahydroborate / 8 h / 55 °C |
|
|
Multi-step reaction with 3 steps
1.1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
1.2: 0.83 h / 40 - 45 °C
2.1: sodium hydroxide; water / methanol / 3 h / pH 6 / Reflux
3.1: sodium tetrahydroborate / 8 h / 55 °C
3.2: 0 °C |
|
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 0.5 h / 10 °C
1.2: 5.5 h / 10 °C
2.1: dichloromethane / 0.25 h / 5 °C
2.2: 0.5 h / 35 - 40 °C
3.1: hydrogenchloride / methanol / 3 h / 55 - 60 °C |
|
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 3.5 h / 10 - 20 °C
2.1: dichloromethane / 0.25 h / 5 °C
2.2: 1 h / 20 °C
3.1: sodium hydroxide / methanol / 2 h / 65 °C |
|
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 5.5 h / 10 - 20 °C
2.1: dichloromethane / 0.25 h / 5 °C
2.2: 1 h / 20 °C
3.1: sodium methylate / methanol / 2 h / 60 °C |
|
|
Multi-step reaction with 3 steps
1: N-chloro-succinimide / dichloromethane; acetonitrile / 2.33 h / -8 °C
2: magnesium / tetrahydrofuran / 2.67 h / 35 - 60 °C
3: iron(III) chloride / tetrahydrofuran / 1 h / 35 °C / Inert atmosphere |
|
|
Multi-step reaction with 3 steps
1: N-chloro-succinimide / dichloromethane; acetonitrile / 2.33 h / -8 °C
2: magnesium / tetrahydrofuran / 2.67 h / 35 - 60 °C
3: iron(III) chloride / tetrahydrofuran / 3 h / 35 °C / Inert atmosphere |
|
|
Multi-step reaction with 3 steps
1: N-Bromosuccinimide / dichloromethane; acetonitrile / 3.5 h / -8 °C
2: magnesium / tetrahydrofuran / 2.75 h / 32 - 62 °C
3: iron(III) chloride / tetrahydrofuran / 3 h / 35 °C / Inert atmosphere |
|
|
Multi-step reaction with 3 steps
1: iron(III) chloride / dichloromethane / 3.33 h / 5 - 20 °C
2: sodium hydroxide / methanol / 3 h / Reflux
3: triacetoxyborohydride / dichloromethane / 12 h / 20 °C |
|
|
Multi-step reaction with 3 steps
1: iron(III) chloride / dichloromethane / 5 - 45 °C
2: sodium hydroxide / methanol / 3 h / Reflux
3: pyridine / butan-1-ol |
|
|
Multi-step reaction with 3 steps
1: iron(III) chloride / dichloromethane / 5 - 45 °C
2: sodium hydroxide / methanol / 3 h / Reflux
3: pyridine; sodium iodide / butanone / 16.25 h / Reflux |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping