|
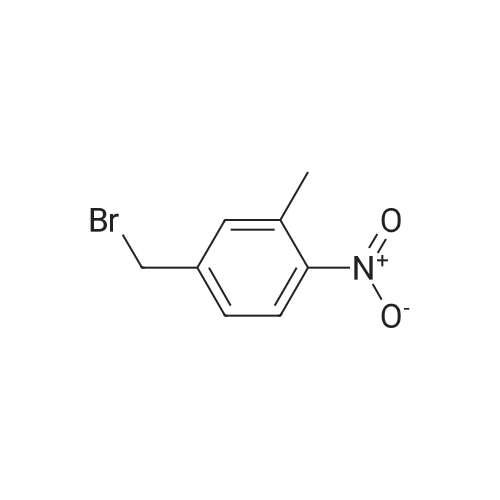
In ethyl acetate; at 20 - 50℃; for 144.0h;Purification / work up; |
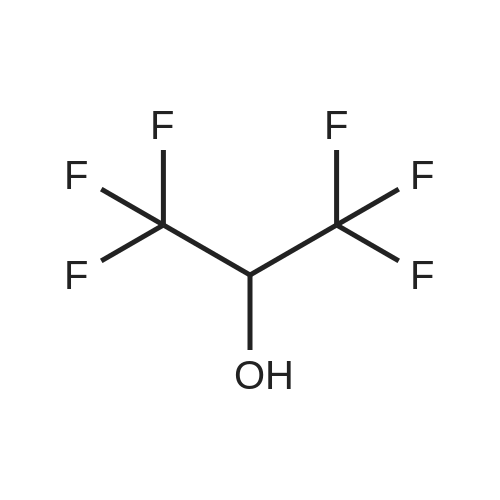
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In isopropyl alcohol; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In N,N-dimethyl-formamide; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In n-heptane; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In toluene; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In cyclohexane; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In 1,4-dioxane; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In 1-methyl-pyrrolidin-2-one; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In butanone; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In acetonitrile; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In ethanol; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In water; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In Isopropyl acetate; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In tetrahydrofuran; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In N,N-dimethyl acetamide; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In acetic acid butyl ester; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In propan-1-ol; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In 4-methyl-2-pentanone; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In acetone; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In methanol; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |
|
In t-BME; at 20 - 50℃; for 144.0h;Purification / work up; |
Example 11: Recrystallization From Various Solvents500muL of each of 24 solvents were added to 50 mg +/- 5 mg of form A, to produce a saturated solution. If complete dissolution was achieved, additional material was added until an excess of solid was present.The vials were capped and placed in a shaking incubator which cycled between ambient temperature and 50 0C, changing every 12 hours. Shaking was continued for 4 days. Inspection of the vials showed that the majority of the 1,1,1,3,3,3- hexafluoropropan-2-ol had evaporated, so an additional 500 muL was added. Inspection after another 2 days showed that this vial now contained only a solution, so additional solid (~30mg) was added.A sample of each slurry was transferred to a glass slide, partially dried either by evaporation or by wicker filtration of any excess solvent and examined by XRPD. Recrystallization from l,l,l,3,3,3-hexafluoro-propan-2-ol afforded crystal form G (see above). Under these conditions, recrystallization from acetone, acetonitrile, THF, DMA, DCM, cyclohexane, heptane, n-butanol, DMF, 1,4-dioxane, ethyl acetate, ethanol, butyl acetate, /-propyl acetate, MEK, methanol, MIBK, propan-1-ol, propan-2-ol, t-BME, toluene, water, or NMP afforded crystal form A. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping