|
With sodium hydroxide; formaldehyd; formic acid; magnesium sulfate; triphenylphosphine; In tetrachloromethane; dichloromethane; water, 1,3-dimethyl-1-propylpyrrolidinium bromide; water; |
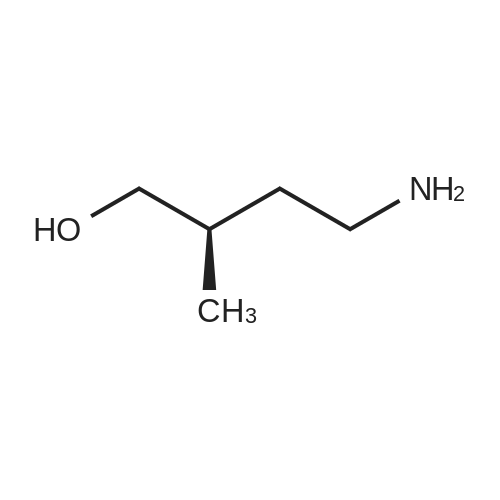
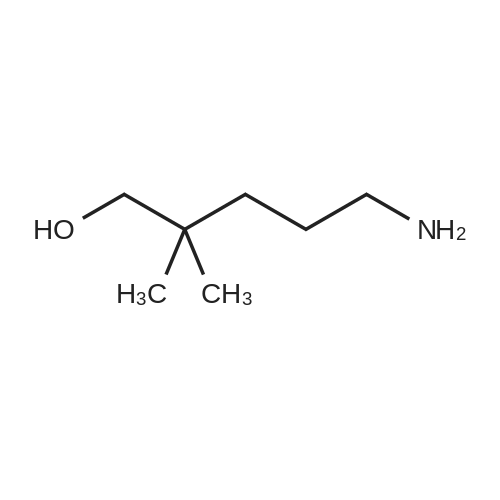
EXAMPLE 1 In this example, a method for producing 1,3-dimethyl-1-propylpyrrolidinium bis(trifluoromethanesulfonyl)amide (abbreviation: 3mP13-TFSA) represented by the structural formula (200) is described. First, <strong>[44565-27-7]4-amino-2-methyl-1-butanol</strong> (24.8 g, 240 mmol) and carbon tetrachloride (111 g, 720 mmol) were mixed under a nitrogen atmosphere at room temperature. Into this mixture, triphenylphosphine (69.2 g, 264 mmol) dissolved in dehydrated dichloromethane (150 ml) was added. Stirring was performed at 40 C. for 1 to 1.5 hours, and then pure water (50 ml) was added to the reacted solution and stirred well. Subsequently, an aqueous phase and a dichloromethane phase were separated. A yellow oily substance was extracted from the dichloromethane phase using pure water (50 ml*2 times). Then, the aqueous phase was washed with toluene (50 ml*3 times), and the solvent was removed by evaporation under reduced pressure to give a yellow oily substance. Sodium hydroxide (19.2 g, 480 mmol) was dissolved in pure water (20 ml), and the sodium hydroxide solution was gradually added to the obtained yellow oily substance, and the mixture was stirred for 12 hours. After that, distillation was performed to give 3-methylpyrrolidine (18.7 g, 219 mmol) which is a colorless transparent liquid. Into formic acid (21.6 g, 470 mmol) being water-cooled, 3-methylpyrrolidine (18.7 g, 219 mmol) was gradually added. Next, a 37% formaldehyde solution (26 ml, 330 mmol) was added to this solution. This solution was heated and refluxed at 100 C., was cooled back to room temperature after a bubble generation, and was stirred for about 30 minutes. Then, the solution was heated and refluxed again for one hour. The formic acid was neutralized with sodium hydroxide, and then the target substance was extracted with diethyl ether and dried using magnesium sulfate, and the solvent was removed by evaporation. Then, distillation was performed, whereby 1,3-dimethylpyrrolidine (13.3 g, 134 mmol) which is a colorless transparent liquid was obtained. Bromopropane (22.3 g, 182 mmol) was added to methylene chloride (10 ml) to which 1,3-dimethylpyrrolidine (12.0 g, 121 mmol) was added, and the mixture was heated and refluxed for 24 hours. The solvent was removed by evaporation, and the obtained white residue was recrystallized in ethanol and ethyl acetate and then dried under reduced pressure at 80 C. for 24 hours, whereby 1,3-dimethyl-1-propylpyrrolidinium bromide (13.9 g, 63.4 mmol) which is a white solid was obtained. In pure water, 1,3-dimethyl-1-propylpyrrolidinium bromide (5.30 g, 23.9 mmol) and lithium bis(trifluoromethanesulfonyl)amide (7.55 g, 26.3 mmol) were mixed and stirred, so that an ionic liquid which is insoluble in water was obtained immediately. After that, the obtained ionic liquid was extracated with methylene chloride and then washed with pure water six times. The solvent was removed by evaporation and dried in vacuum at 100 C., so that 1,3-dimethyl-1-propylpyrrolidinium bis(trifluoromethanesulfonyl)amide (9.37 g, 22.2 mmol) was obtained. The compound obtained through the above steps was identified as 1,3-dimethyl-1-propylpyrrolidinium bis(trifluoromethanesulfonyl)amide which is a target substance by using a nuclear magnetic resonance (NMR) and mass spectrometry. 1H NMR data of the obtained compound is shown below. 1H-NMR (CDCl3, 400 MHz, 298 K): delta=0.97-1.05 (3H), 1.15-1.21 (3H), 1.67-1.99 (3H), 2.28-2.48 (1H), 2.58-2.78 (1H), 2.94-3.08 (1H), 3.06, 3.13 (3H), 3.18-3.34 (2H), 3.47-3.87 (3H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping