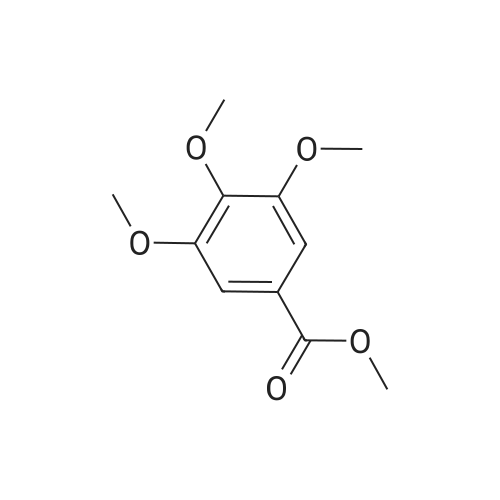
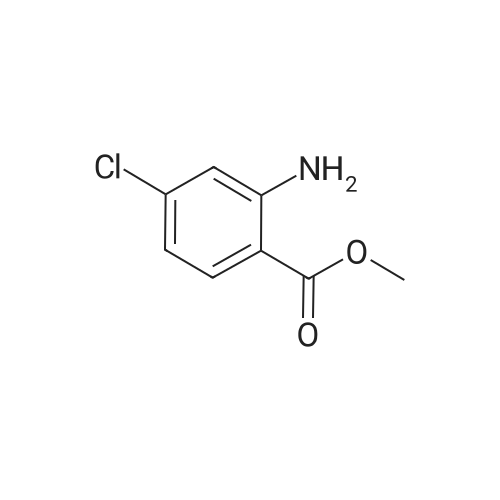
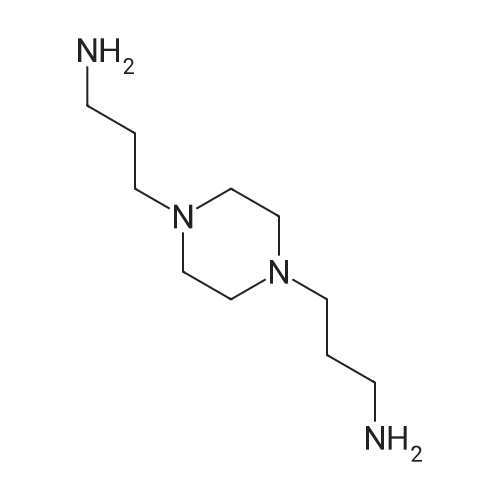
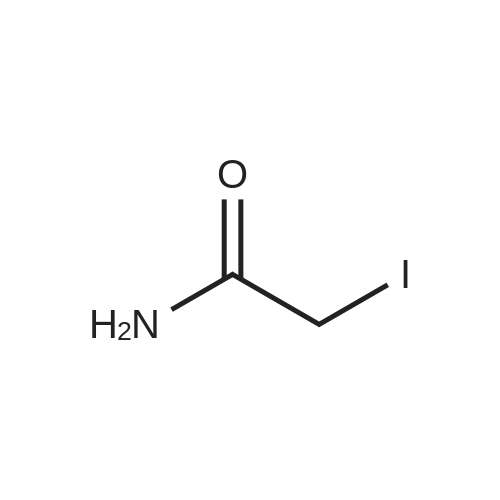
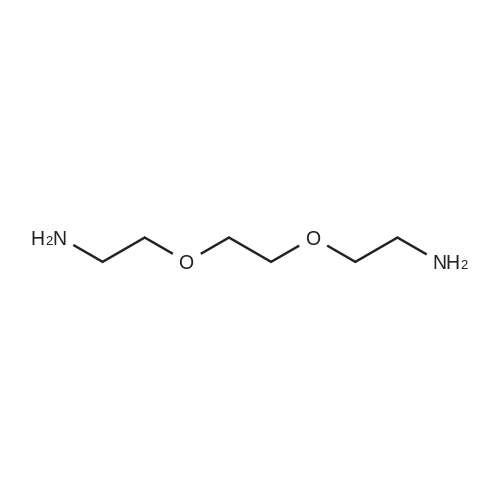
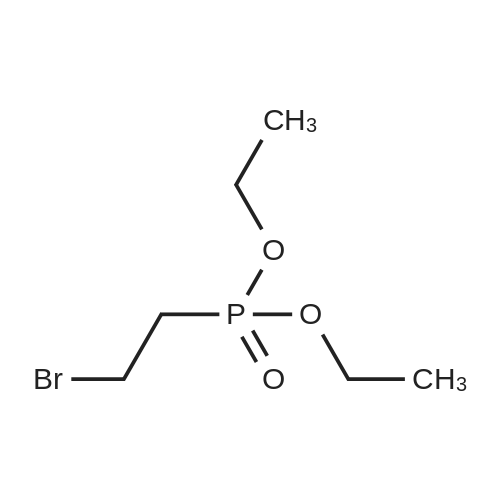
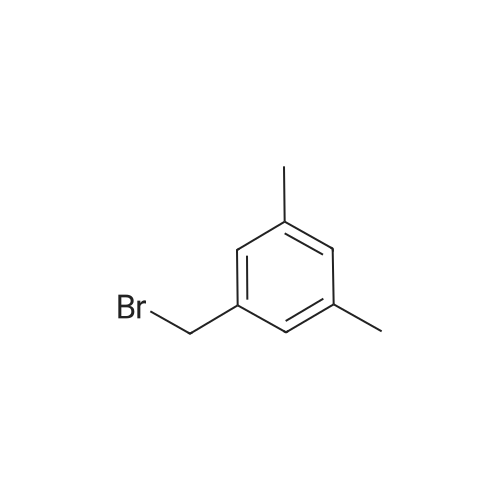
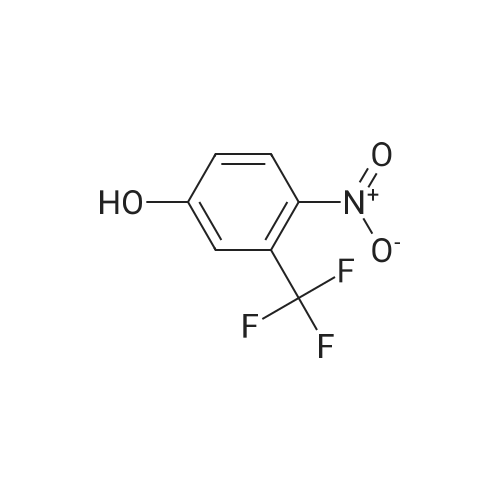
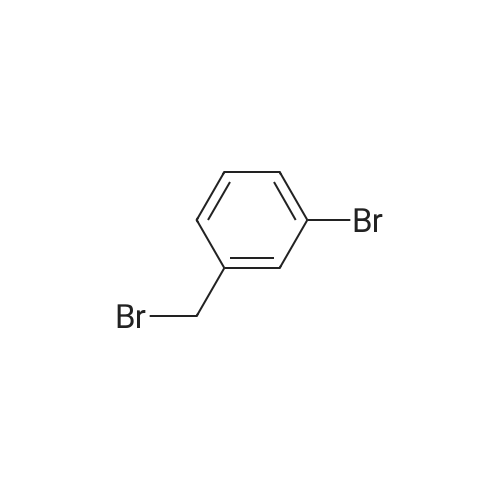
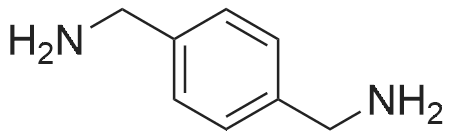
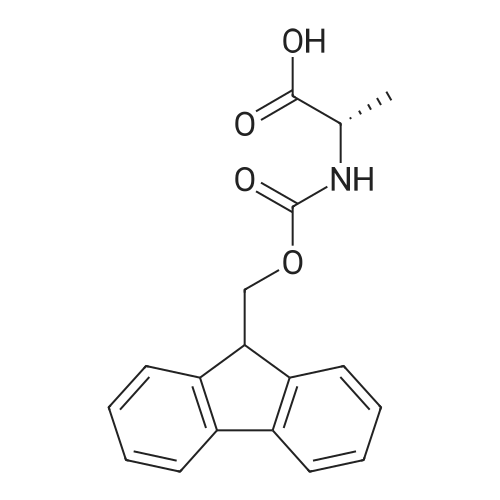
|
With piperidine; Carbonyldiimidazole; tin-2-ethylhexanoate dihydrate; potassium tert-butylate; water; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; diisopropyl-carbodiimide; dibromotriphenylphosphorane; In DMF (N,N-dimethyl-formamide); dichloromethane; N,N-dimethyl acetamide; 1,2-dichloro-ethane;Combinatorial reaction / High throughput screening (HTS); |
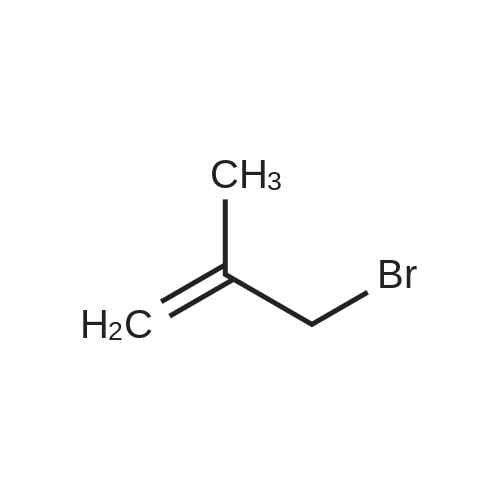
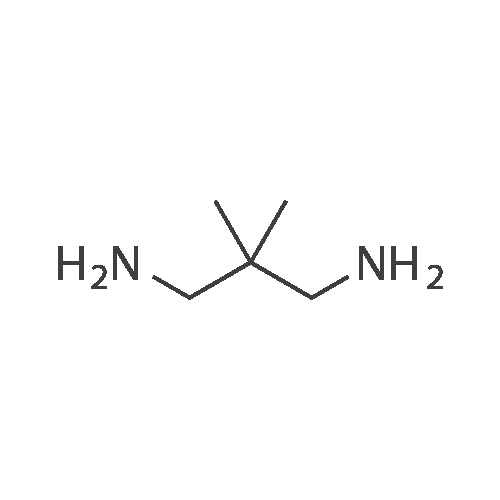
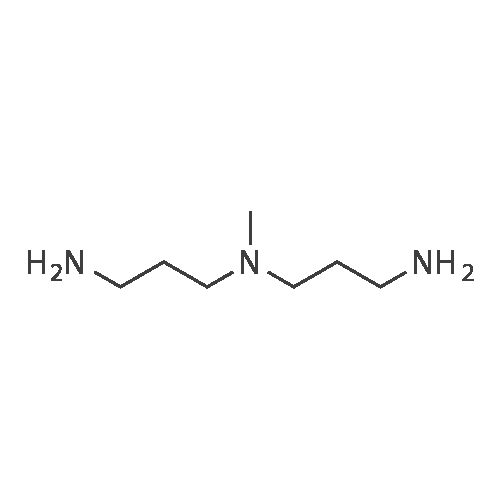
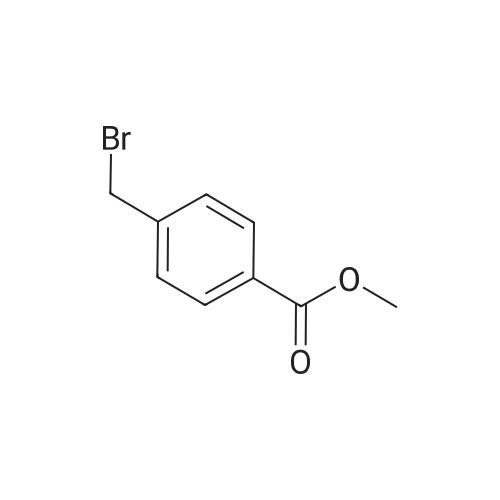
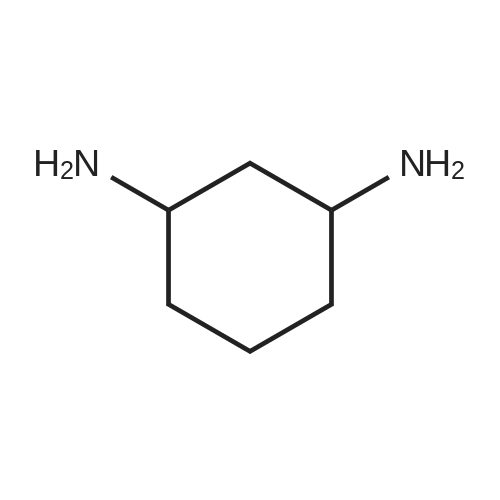
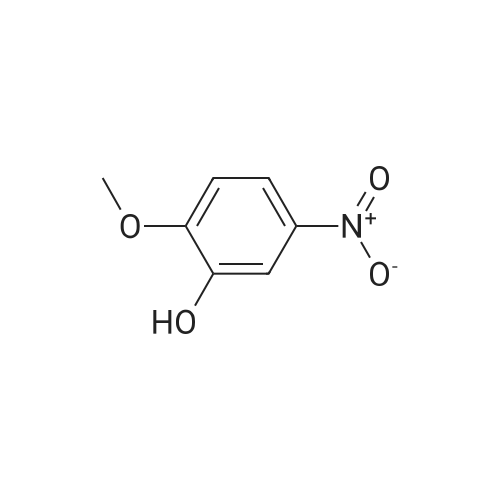
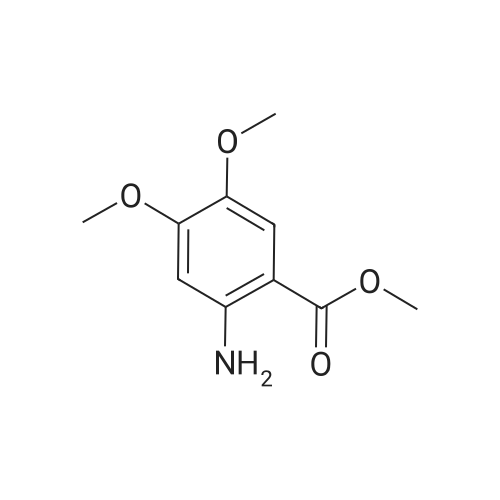
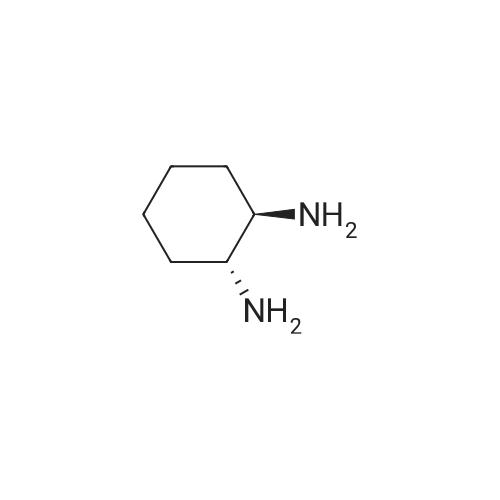
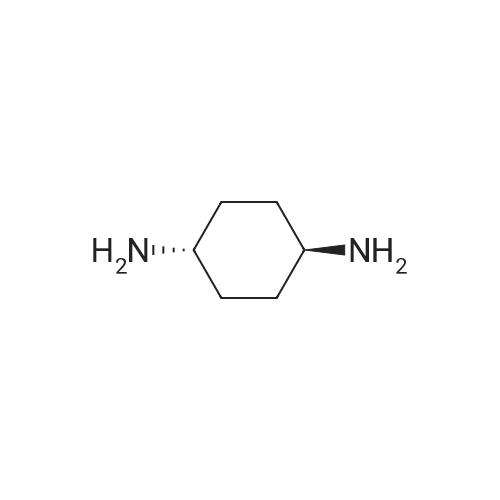
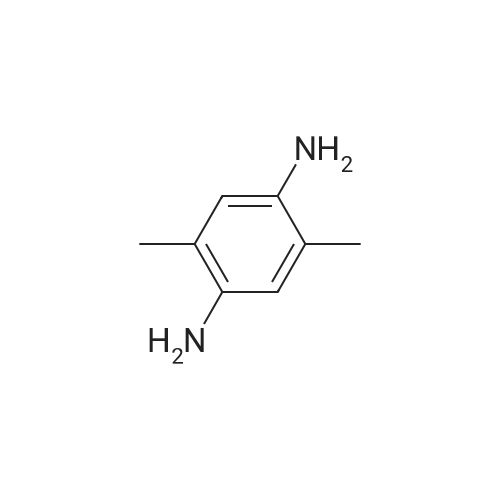
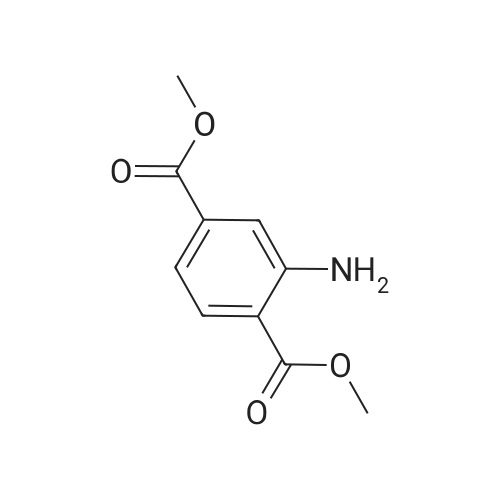
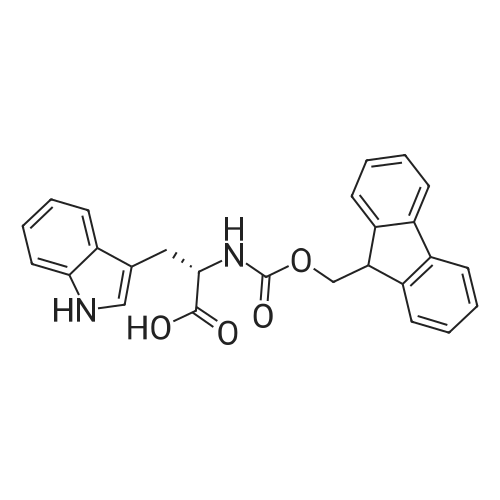
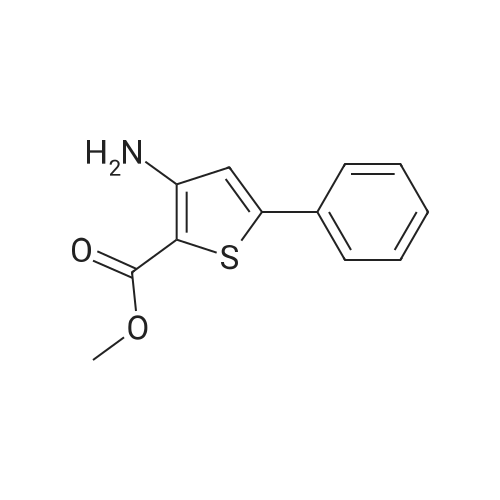
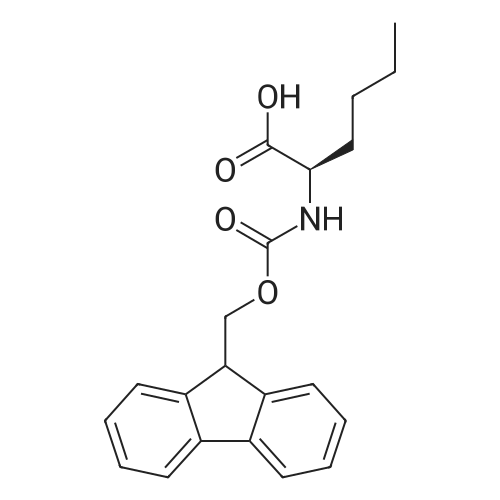
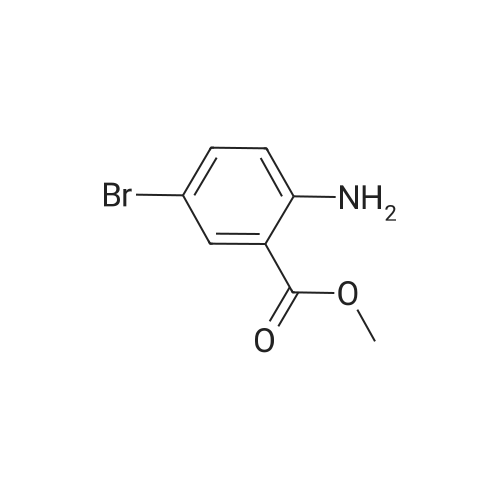
EXAMPLE 1 [0213] This example shows the synthesis of a combinatorial library of thioquinazolinone derivatives. [0214] Step 1a: Preparation of Wang Bromide Resin [0215] 40 tea bags containing 2 g each of Wang resin (80 g, 120 mmol) was taken in a 5 L PP container. A solution of triphenylphosphine dibromide (152 g, 0.15 M, 3 eq., 360 mmol) in 2000 ml DCM was added and the solution was shaken at room temperature overnight. The resin was sequentially washed with DCM (4×, 1.5 L each) and diethylether (6×, 1.5 L each) and dried under vacuum, to give the bromo wang resin. [0216] Step 1b: Loading of the Nitrophenol on Bromo Wang [0217] 20 g of the Bromo wang resin (1.5 meq/g) was taken in a 2 L wide-mouthed glass container and 1000 mL DMA was addded to it followed by the addition of the nitro phenol (10 eq., 0.3M, 300 mmol). Potasium t-butoxide (33.46 g, 10 eq., 300 mmol) was then added to it and the bottles were heated at 50 C. overnight. The bags were washed alternatively with DMF (500 mL) and DCM (500 mL) 3 cycles followed by 6 cycles of MeOH (500 mL). The tea bags were then dried overnight in air. The following nitrophenols were used: [0218] 2-METHYL-5-NITROPHENOL [0219] 5-HYDROXY-2-NITROBENZOTRIFLUORIDE [0220] 3-METHYL-4-NITROPHENOL [0221] 2-METHOXY-5-NITROPHENOL [0222] M-NITROPHENOL [0223] Step 1c: Reduction of the Nitro Group to Amine [0224] A 2.0 M solution of tin-2-ethylhexanoate dihydrate was prepared in DMF containing 0.5% H2O. The tea bags were added and the solution is heated at 50 C. for 40 hours. After cooling the bags are washed with DMF/10% HOAc (3×), DMF (3×), 5% DIEA/DCM (2×), DCM (2×) and MeOH (2×) and dried in air overnight. [0225] Step 1d: Coupling N-FMOC Protected Amino Acid to Wang Resin. [0226] 20 g of Wang resin (1.5 meq/g) was placed in a porous polypropylene packet (Tea-bag, 60 mm×60 mm, 65mu) and taken in a 1000 mL plastic bottle. DMF (300 mL), DCM (300 mL), FMOC-Cyclohexyl alanine (70.82 g, 6 eq., 0.3M, 180 mmol), DIC (22.71 g, 6 eq., 180 mmol), HOBt (24.32 g, 6 eq., 180 mmol) were added sequentially. After shaking for 12 hours, the packet was washed alternatively with DMF (500 mL) and DCM (500 mL) 3 cycles followed by 6 cycles of MeOH (500 mL). The packet was then dried overnight in air. The tea bags containing the amino acids were then treated with 20% piperidine/DMF for 2 h at room temperature to deblock the FMOC group. The following amino acids were used: [0227] FMOC-GLY-OH [0228] FMOC-ALA-OH [0229] FMOC-L-ISOLEUCINE [0230] FMOC-L-PHENYLALANINE [0231] FMOC-D-NLE-OH [0232] FMOC-CHA-OH [0233] FMOC-L-TRYPTOPHAN [0234] Step 1e: Coupling of the Diamines to Wang Resin [0235] 20 g of Wang resin (1.5 meq/g) was placed in a porous polypropylene packet (Tea-bag, 60 mm×60 mm, 65mu) and taken in a 1000 mL Nalgene bottle. 600 mL of DCM was added followed by the addition of the carbonyl diimidazole (29.9 g, 6 eq., 0.3M, 180 mmol) and the flasks were shaken at room temperature for 3 hours after which they were decanted and washed with DCM (2×, 600 mL). To these Nalgene bottles were added the diamines (6 eq., 0.4M, 180 mmol) in 450 mL of DCM (0.4M) and they were shaken at room temperature overnight. The diamines used were as follows: [0236] 2,2-DIMETHYL-1,3-PROPANEDIAMINE [0237] 1,3-CYCLOHEXANEDIAMINE [0238] (1R,2R)-(-)-1,2-DIAMINOCYCLOHEXANE [0239] TRANS-1,4-DIAMINOCYCLOHEXANE [0240] P-XYLYLENEDIAMINE [0241] 1,4-BIS(3-AMINOPROPYL)PIPERAZINE [0242] ETHYLENEDIAMINE [0243] 1,3-DIAMINOPROPANE [0244] 1,8-DIAMINO-3,6-DIOXAOCTANE [0245] 1,4-DIAMINOBUTANE [0246] 1,5-DIAMINOPENTANE [0247] 1,6-HEXANEDIAMINE [0248] N,N-BIS(3-AMINOPROPYL)METHYLAMINE [0249] 2,2'-THIOBIS(ETHYLAMINE) [0250] 2,5-DIMETHYL-1,4-PHENYLENEDIAMINE [0251] After shaking overnight, the packets was washed alternatively with DMF (500 mL) and DCM (500 mL) 3 cycles followed by 6 cycles of MeOH (500 mL). The packet was then dried in air. [0252] Step 2: Formation of the Isothiocyanate [0253] The o-amino benzoate ester (136 g, 10 eq., 900 mmol) was taken in a 5 L wide-mouthed glass bottle and 2.7 L of dichloroethane was added to it (0.3M). The following esters were used: [0254] METHYL ANTHRANILATE [0255] METHYL 2-AMINO-4-CHLOROBENZOATE [0256] 2-AMINO-4,5-DIMETHOXYBENZOIC ACID [0257] METHYL ESTER [0258] METHYL 3,4,5-TRIMETHOXYANTHRANILATE [0259] DIMETHYL AMINOTEREPHTHALATE [0260] METHYL 2-AMINO-5-BROMOBENZOATE [0261] METHYL 3-AMINOTHIOPHENE-2-CARBOXYLATE [0262] METHYL 3-AMINO-5-PHENYLTHIOPHENE-2-CARBOXYLATE [0263] Thiocarbonyl diimidazole (160 g, 10 eq., 900 mmol) was added to it and the solution was heated at 55 C. overnight to form the isothiocyanate. [0264] Step 3: Formation of the Thioquinazolinone [0265] The next day the tea bags containing the amino acids, diamines and the amino phenols on wang resin (90 mmol) was added to the isothiocyanate solution from reaction 2 and the glass bottles were heated at 55 C. overnight. After cooling the bags was washed alternatively with DMF (2000 mL) and DCM (2000 mL) 3 cycles followed by 6 cycles of MeOH... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping