Alternatived Products of [ 52565-83-0 ]
Product Details of [ 52565-83-0 ]
| CAS No. : | 52565-83-0 |
MDL No. : | MFCD06014354 |
| Formula : |
C6H9N3O3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
171.15
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 52565-83-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 52565-83-0 ]
- 1
-
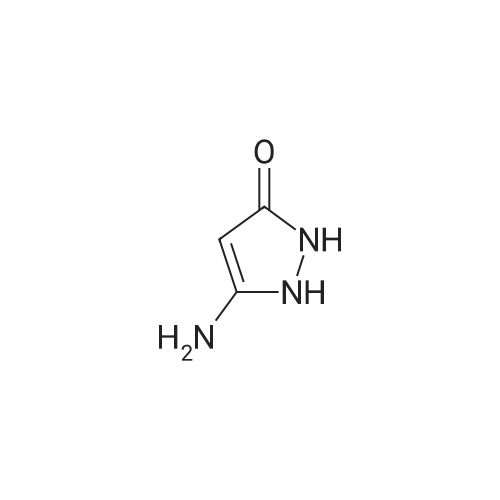 [ 28491-52-3 ]
[ 28491-52-3 ]

-
 [ 541-41-3 ]
[ 541-41-3 ]

-
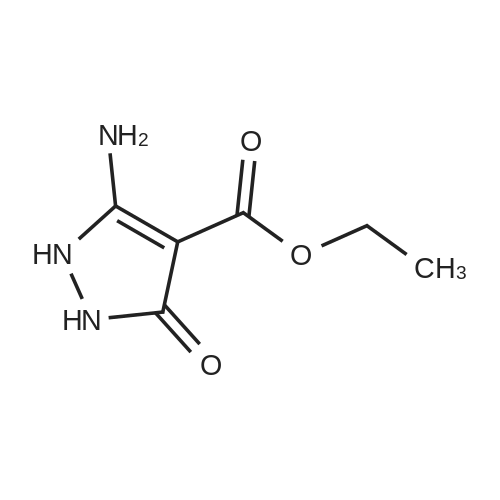 [ 52565-83-0 ]
[ 52565-83-0 ]
- 3
-
 [ 52565-83-0 ]
[ 52565-83-0 ]

-
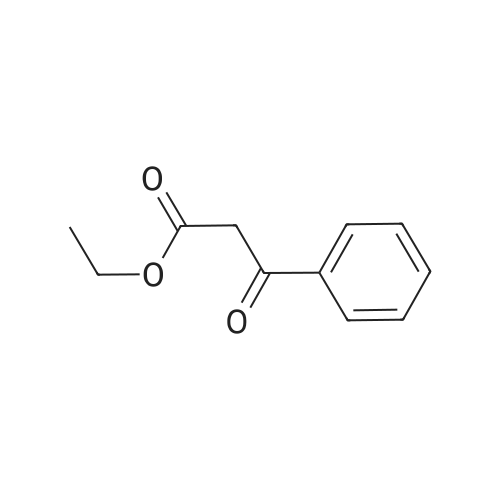 [ 94-02-0 ]
[ 94-02-0 ]

-
 [ 1257439-26-1 ]
[ 1257439-26-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 68% |
at 150 - 160℃; for 4.5h; |
A mixture of ethyl delta-amino-S-oxo^.S-dihydro-I H-pyrazole^-carboxylate (0.17 g, 1.0 mmol) and ethyl 3-phenyl-3-oxopropanoate (0.73 g, 3.68 mmol) is stirred for 4.5 h (TLC control) at 150-160 0C. The mixture is then cooled to rt and stirred for 10 min with Et2theta and filtered. The resulting solid is washed with Et2theta and MeOH and dried to give 0.2 g (68%) of the title compound. 1H-NMR (400 MHz, DMSO-d6), delta (ppm): 1.28 (t, 7 Hz, 3H); 4.24 (q, 7 Hz, 2H); 6.20 (s, 1 H); 7.54-7.57 (m, 3H); 7.72-7.75 (m, 2H) and 11.07 (br s, 1 H) and 11.92 (s, 1 H). LC-MS m/z 300 (M+1 ). |
- 4
-
 [ 52565-83-0 ]
[ 52565-83-0 ]

-
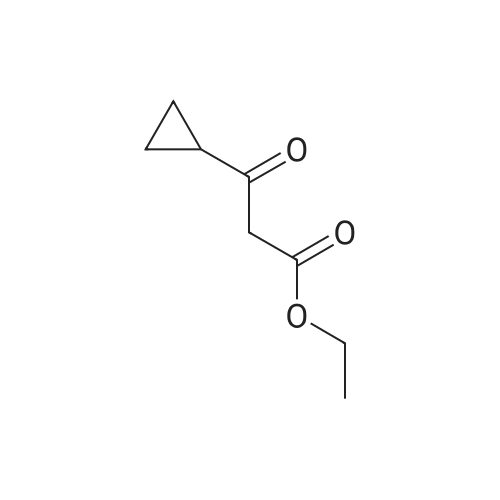 [ 24922-02-9 ]
[ 24922-02-9 ]

-
 [ 1257440-16-6 ]
[ 1257440-16-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 63% |
In acetic acid; at 85℃; for 24.0h; |
A mixture of ethyl 5-amino-3-oxo-2,3-dihydro-1 H-pyrazole-4-carboxylate (250 mg, 1.46 mmol), ethyl 3-cyclopropyl-3-oxopropanoate (251 mg, 1.61 mmol, 1.10 equiv.) in acetic acid (2 ml) is stirred for 24 h at 85 0C. The mixture is allowed to cool then it is concentrated in vacuo. The residue is suspended in ether and filtered off to leave 245 mg (63%) of the product as a solid. LC-MS m/z 262 (M-H); 1H-NMR (400 MHz, DMSO-d6): delta (ppm) 0.89-0.95 (2H, m), 1.03-1.11 (2H, m), 1.29 (3H1 t), 2.27-2.36 (1 H, m), 4.27 (2H1 q), 5.43 (1 H, s), 11.27 (1 H, br s), 11.48 (1 H, br s). |
- 5
-
 [ 52565-83-0 ]
[ 52565-83-0 ]

-
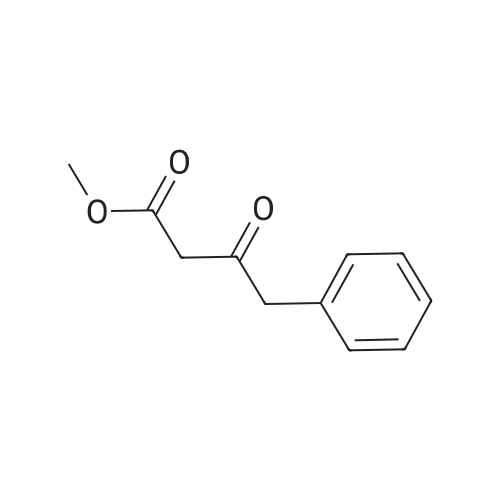 [ 37779-49-0 ]
[ 37779-49-0 ]

-
 [ 1257440-14-4 ]
[ 1257440-14-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 71% |
In acetic acid; at 80℃; for 2h; |
Methyl 3-oxo-4-phenylbutanoate (404 mg, 2.10 mmol, 1.2 equiv.) is added to a suspension/solution of ethyl 5-amino-3-oxo-2,3-dihydro-1 H-pyrazole-4-carboxylate (300 mg, 1.76 mmol) in acetic acid (7 ml). The mixture is heated at 80 0C for 2 h. Then it is allowed too cool, diluted with water and concentrated to dryness. The orange residue is triturated with MeOH and filtered to afford 108 mg of an off-white powder. The filtrate is again concentrated and the residue is triturated with EtOH and filtered to afford a total yield of 389.6 mg (71%) of an off-white powder.LC-MS m/z 312 (M-H); 1H-NMR (400 MHz1 DMSO-d6): delta (ppm)_1.27 (3H, t), 4.08 (2H1 s), 4.25 (2H, q), 5.71 (1 H1 s), 7.25-7.31 (1 H1 m), 7.33-7.40 (4H, m), 11.45 (1 H, br s), 11.45 (1 H. br s). |
- 6
-
 [ 52565-83-0 ]
[ 52565-83-0 ]

-
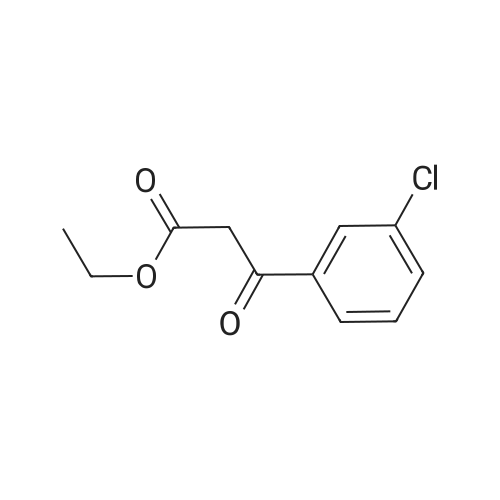 [ 33167-21-4 ]
[ 33167-21-4 ]

-
 [ 1257440-20-2 ]
[ 1257440-20-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 28% |
In acetic acid; at 100℃; for 48h; |
Ethyl 5-amino-3-oxo-2,3-dihydro-1 H-pyrazole-4-carboxylate (12.25 g, 71.5 mmol) is dispersed in acetic acid (70 ml) and <strong>[33167-21-4]ethyl 3-(3-chlorophenyl)-3-oxopropanoate</strong> (19.46 g, 86 mmol, 1.2 equiv.) is added. The mixture is heated to 100 0C for 2 days. The resulting suspension is filtered off and washed with diisopropyl ether to give an off-white solid. The solid is stirred with acetic acid (30 ml) at 100 0C for 5 min. Hot filtration affords 6.7 g (28%) of the product as an off-white solid.LC-MS m/z 332/334 (M-H, Cl isotope pattern); 1H-NMR (400 MHz1 DMSO-d6): delta (ppm) 1.32 (3H1 t), 4.26 (2H, q), 6.27 (1 H, s), 7.59 (1 H1 t), 7.65-7.69 (1 H1 m), 7.70-7.75 (1 H1 m), 7.83 (1 H1 1), 11.40 (1 H, s), 11.66 (1 H1 br s). |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping











