Alternatived Products of [ 53389-98-3 ]
Product Details of [ 53389-98-3 ]
| CAS No. : | 53389-98-3 |
MDL No. : | MFCD06625273 |
| Formula : |
C15H14O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
226.27
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 53389-98-3 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 53389-98-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 53389-98-3 ]
- 1
-
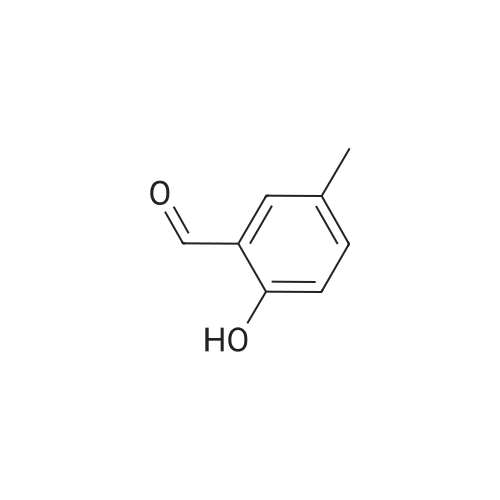 [ 613-84-3 ]
[ 613-84-3 ]

-
 [ 100-39-0 ]
[ 100-39-0 ]

-
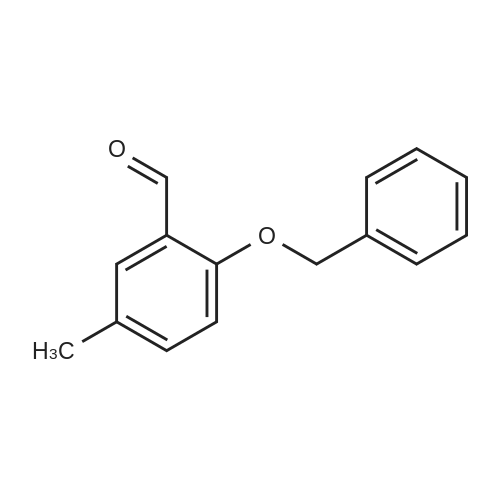 [ 53389-98-3 ]
[ 53389-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 100% |
With sodium hydride In N,N-dimethyl-formamide; mineral oil Inert atmosphere; |
|
| 84% |
With potassium carbonate In N,N-dimethyl-formamide at 65℃; for 6h; |
Step 1: Synthesis of 2-(benzyloxy)-5-methylbenzaldehyde.
2-Hydroxy-5-methylbenzaldehyde (5.00 g, 36 mmol) was dissolved in dimethylformamide(30 ml); K2CO3 (6.07 g, 44 mmol) and benzyl bromide(4.36 ml, 36 mmol) were added; and the reaction mixture waswarmed to 65°C and stirred for 6 hours. The reaction mixture wascooled to room temperature and poured into water (500 ml). Theprecipitate was filtered, washed with water (100 ml), and dried togive 2-(benzyloxy)-5-methylbenzaldehyde (7.0 g, 84% yield) asa white solid. |
| 82% |
Stage #1: 2-hydroxy-5-methylbenzaldehyde With 18-crown-6 ether; potassium carbonate In acetone at 20℃; for 0.5h;
Stage #2: benzyl bromide In acetone for 3h; Reflux; |
|
|
With potassium hydroxide 1.) Aliquat 336, 85 deg C, 10 min, 2.) Aliquat 336, 5 h; Yield given. Multistep reaction; |
|
|
With potassium carbonate In N,N-dimethyl-formamide |
|
|
With caesium carbonate In acetone at 20℃; |
4.5 Protection of substituted salicylaldehydes with benzyl group
General procedure: In a typical experiment: 5-chlorosalicylaldehyde (20) (1.0 g/6.4 mmol) was placed in a two-necked round-bottom flask and then it was added Cs2CO3 (3.13 g/9.6 mmol), benzyl bromide (1.64 g/9.6 mmol) and 50 mL of acetone. The mixture was allowed to stir at room temperature overnight. The product was then filtered and washed with acetone. The solvent was evaporated and the crude product purified by column chromatography (silica gel, n-hexane/ethyl acetate 95:5). Compound 24a was obtained as white solid (1.42 g/90%). |
|
With potassium carbonate In acetone at 20℃; for 8h; Schlenk technique; Glovebox; |
|
|
Stage #1: 2-hydroxy-5-methylbenzaldehyde With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.5h; Inert atmosphere;
Stage #2: benzyl bromide With tetra-(n-butyl)ammonium iodide In tetrahydrofuran; mineral oil for 3h; Inert atmosphere; |
|

Reference:
[1]Mori, Keiji; Kawasaki, Taro; Sueoka, Shosaku; Akiyama, Takahiko
[Organic Letters, 2010, vol. 12, # 8, p. 1732 - 1735]
[2]Sato, Tomomi; Baker, Jillian; Warne, Tony; Brown, Giles A.; Leslie, Andrew G.W.; Congreve, Miles; Tate, Christopher G.
[Molecular Pharmacology, 2015, vol. 88, # 6, p. 1024 - 1034]
[3]Zullo, Valerio; Iuliano, Anna
[European Journal of Organic Chemistry, 2019, vol. 2019, # 6, p. 1377 - 1384]
[4]Meier, H.; Kretzschmann, H.; Lang, M.
[Journal fur Praktische Chemie - Chemiker-Zeitung, 1994, vol. 336, # 2, p. 121 - 128]
[5]Chen, Han; Fan, Yun-Hua; Natarajan, Amarnath; Guo, Yuhong; Iyasere, Julia; Harbinski, Fred; Luus, Lia; Christ, William; Aktas, Huseyin; Halperin, Jose A.
[Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 21, p. 5401 - 5405]
[6]Azevedo, Carlos M.G.; Afonso, Carlos M.M.; Sousa, Diana; Lima, Raquel T.; Helena Vasconcelos; Pedro, Madalena; Barbosa, João; Corrêa, Arlene G.; Reis, Salette; Pinto, Madalena M.M.
[Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 11, p. 2941 - 2959]
[7]Wang, Xiaoming; Wang, Xubin; Guo, Peihua; Wang, Zheng; Ding, Kuiling
[Advanced Synthesis and Catalysis, 2013, vol. 355, # 14-15, p. 2900 - 2907]
[8]Shen, Hongjuan; Fu, Junkai; Yuan, Hao; Gong, Jianxian; Yang, Zhen
[Journal of Organic Chemistry, 2016, vol. 81, # 21, p. 10180 - 10192]
- 2
-
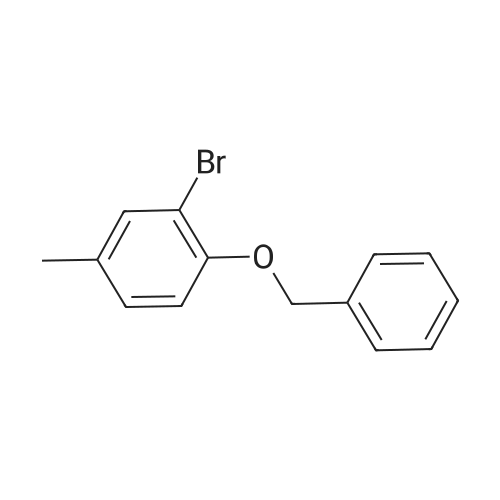 [ 2830-53-7 ]
[ 2830-53-7 ]

-
 [ 33513-42-7 ]
[ 33513-42-7 ]

-
 [ 53389-98-3 ]
[ 53389-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 100% |
Stage #1: 1-(benzyloxy)-2-bromo-4-methylbenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h;
Stage #2: N,N-dimethyl-formamide In tetrahydrofuran; hexane at -78 - 20℃; for 12h; Further stages.; |
|
- 3
-
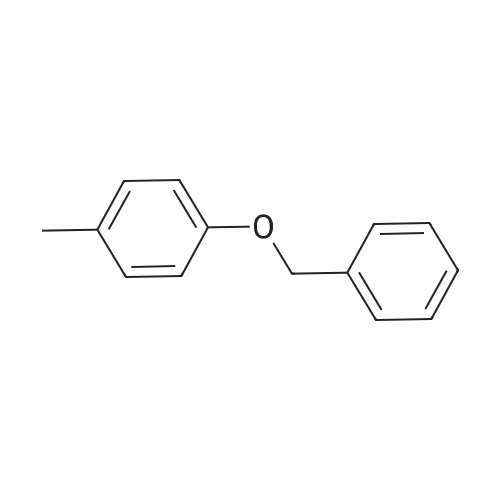 [ 834-25-3 ]
[ 834-25-3 ]

-
 [ 4885-02-3 ]
[ 4885-02-3 ]

-
 [ 53389-98-3 ]
[ 53389-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 76% |
With silver trifluoromethanesulfonate In dichloromethane at -78℃; for 0.166667h; Inert atmosphere; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








