Alternatived Products of [ 54231-44-6 ]
Product Details of [ 54231-44-6 ]
| CAS No. : | 54231-44-6 |
MDL No. : | MFCD12923070 |
| Formula : |
C9H11ClN2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
198.65
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 54231-44-6 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 54231-44-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 54231-44-6 ]
- 1
-
 [ 110-91-8 ]
[ 110-91-8 ]

-
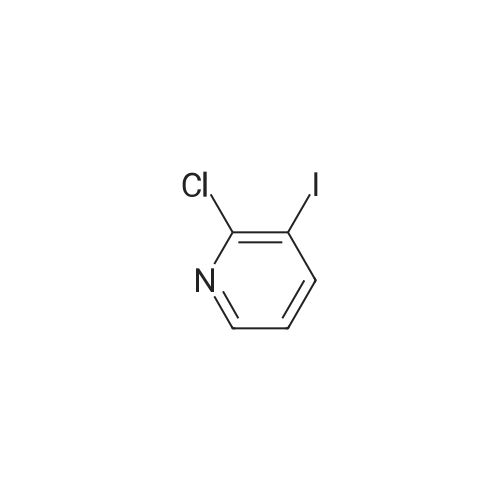 [ 78607-36-0 ]
[ 78607-36-0 ]

-
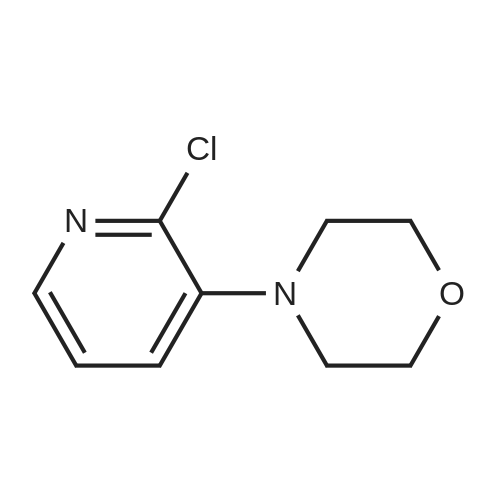 [ 54231-44-6 ]
[ 54231-44-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 71% |
With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; In 1,4-dioxane; at 90℃; for 48h;Inert atmosphere; |
A suspension of <strong>[78607-36-0]2-chloro-3-iodopyridine</strong> (1) (1.07g, 4.46mmol), morpholine (0.43ml_, 4.90mmol), Cs2C03 (2.90gmg, 8.92mmol) and Xantphos (260mg, 0.4mmol) in 1 ,4-dioxane (50mL) was purged with Ar(g) for 0.5h. Pd2(dba)3 (204mg, 0.22mmol) was added and the reaction mixture was heated up to 90C for 2d. Once cooled down to rt, it was poured into a mixture of H20 (50ml_) and brine (50ml_), then extracted with EtOAc (3 x 50ml_). The combined organics were washed with brine (50ml_), dried over MgS04, filtered and concentrated in vacuo. Purification by silica gel column chromatography with hexane/EtOAc (1 :0-7:3) yielded (2) as a solid (628mg, 71 %). |
| 64% |
With caesium carbonate;palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In toluene; at 120℃; for 16h;Inert atmosphere; |
A 2-dram vial equipped with a stirbar was charged with Pd(OAc)2 (0.234 g, 1.044 mmol) and rac-BINAP (0.683 g, 1.096 mmol) as solids. Toluene (4 mL) was added via syringe, and the mixture was stirred for 10 min while being degassed with argon, and until a uniform tan suspension resulted. A 100-mL round-bottom flask was charged with <strong>[78607-36-0]2-chloro-3-iodopyridine</strong> (1.25 g, 5.22 mmol) and Cs2C03 (7.14 g, 21.93 mmol). Toluene (34.8 mL) was added followed by morpholine (0.542 ml, 6.26 mmol). This solution was degassed for 1 min under argon, and then the catalyst ligand suspension was added via pasteur pipette. A reflux condenser was attached to the flask, and the reaction mixture was refiuxed at 120 C for 16 h. The reaction mixture was concentrated in vacuo, and then diluted with CH2C12 (150 mL). The organics were washed with 2x 100 mL brine, dried over Na2S04, filtered, and concentrated in vacuo. The material was purified by gradient elution on silica gel (5 to 75% [5% MeOH/EtOAc] in hexanes) to afford the title compound as light yellow solid (700 mg, 64%). |
- 2
-
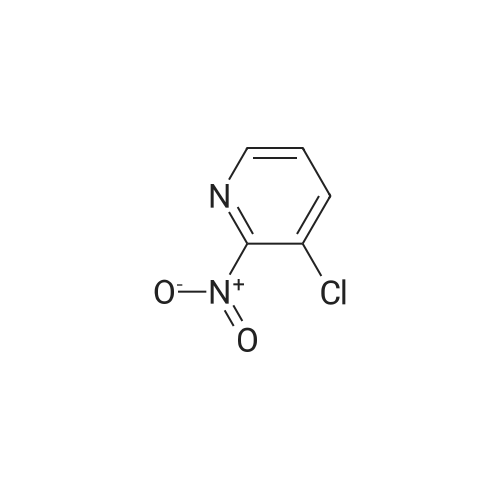 [ 54231-32-2 ]
[ 54231-32-2 ]

-
 [ 54231-44-6 ]
[ 54231-44-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: methanol / Heating
2: aq. HCl / Heating |
|
- 3
-
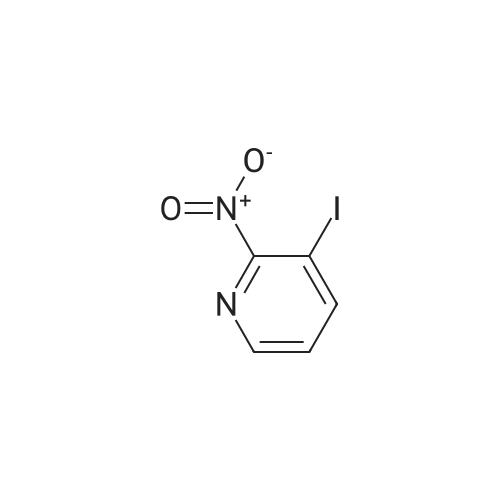 [ 54231-34-4 ]
[ 54231-34-4 ]

-
 [ 54231-44-6 ]
[ 54231-44-6 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







