Alternatived Products of [ 573-98-8 ]
Product Details of [ 573-98-8 ]
| CAS No. : | 573-98-8 |
MDL No. : | MFCD00004035 |
| Formula : |
C12H12
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
156.22
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 573-98-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 573-98-8 ]
- 1
-
 [ 56-23-5 ]
[ 56-23-5 ]

-
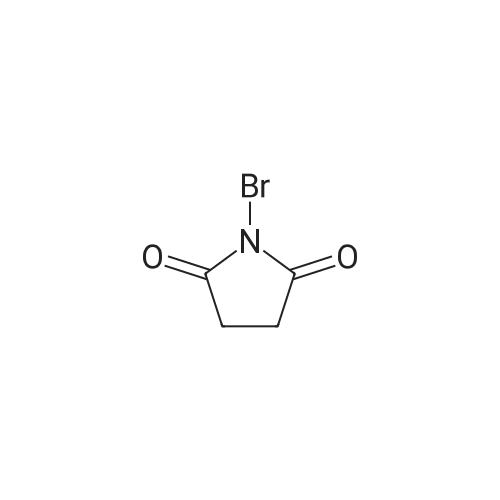 [ 128-08-5 ]
[ 128-08-5 ]

-
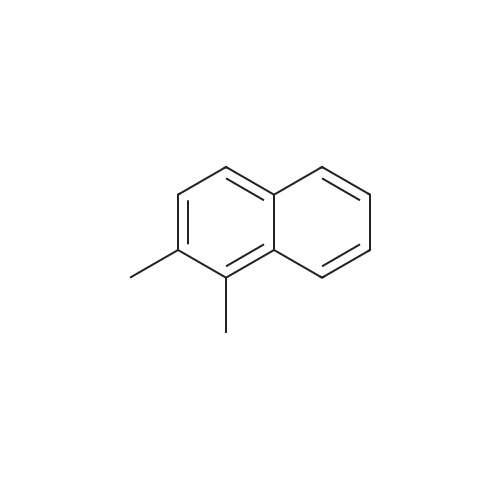 [ 573-98-8 ]
[ 573-98-8 ]

-
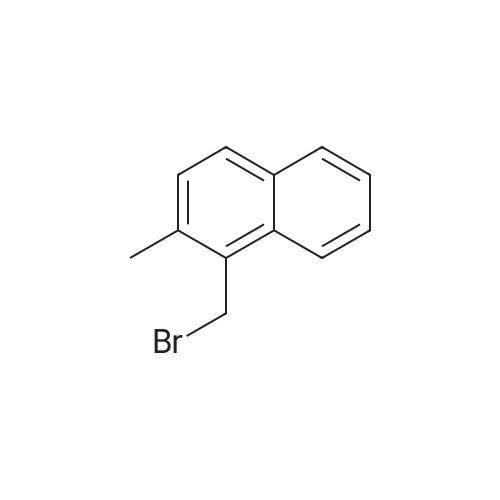 [ 61172-29-0 ]
[ 61172-29-0 ]
- 2
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
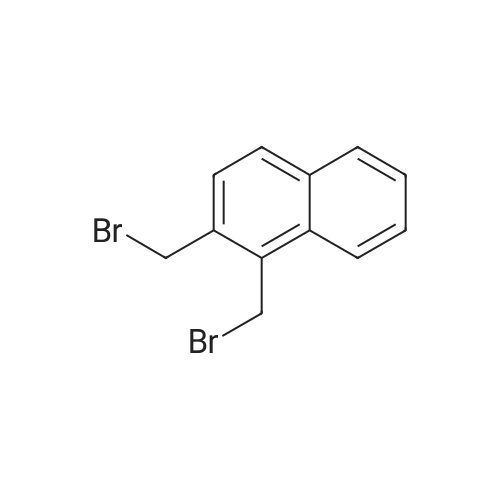 [ 59882-98-3 ]
[ 59882-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 100% |
With N-Bromosuccinimide; In tetrachloromethane;Irradiation; |
General procedure (a): A solution of 1,2-dimethylaryle 2b-e,h (10 mmol) and finely pulverised N-bromosuccinimide (NBS 3.68 g, 21 mmol, 2.1 equiv) in CCl4 (40-80 mL) was irradiated with HPK125 mercury lamp for 1-2 h with good stirring (tlc or 1H NMR monitoring). The reaction mixture was diluted with CH2Cl2, washed with H2O or 2 N aqueous NH4Cl solution and dried over MgSO4. The solvent was evaporated to give quantitatively 5b-e,h which was used without further purification. |
|
With N-Bromosuccinimide; In tetrachloromethane; for 1h;Heating / reflux; |
[0131] To a stirred solution of 1,2-dimethylnaphthalene (5) (1.56 g, 1 equiv.) in CCl4 (10 mL) was added NBS (3.56 g, 2 equiv.). The reaction mixture was then refluxed for 1 h. After cooling to rt, the mixture was filtered and washed with CHCl3. The filtrate was washed with H2O and brine, dried over MgSO4 and concentrated under reduced pressure to yield a crude solid. This solid was washed with a small portion of toluene/CHCl3 to yield the purified dibromide (6). To a suspension of Na2NCN (516 mg, 2 equiv.) in DMSO (30 mL) was added the dibromide (6) (924 mg, 1 equiv.). The suspension was stirred at rt for 1 h at which point the mixture was poured into H12O. The aqueous phase was extracted with Et2O (3×) and the combined organic extracts were washed with H2O and brine, dried over MgSO4 and concentrated under reduced pressure. The resultant residue was purified by flash chromatography (gradient elution: 10% EtOAc in hexane to 20% EtOAc in hexane) to afford the solid 2-cyano-2,3-dihydro-1H-benzo[e]isoindole (7). |
Reference:
[1]Bioorganic and Medicinal Chemistry,2011,vol. 19,p. 5716 - 5733
[2]Journal of Medicinal Chemistry,1997,vol. 40,p. 1720 - 1725
[3]Synthesis,2011,p. 2945 - 2950
[4]Tetrahedron,2008,vol. 64,p. 4275 - 4286
[5]Bioorganic and Medicinal Chemistry,2009,vol. 17,p. 3568 - 3571
[6]Chemische Berichte,1956,vol. 89,p. 708,711
[7]Synthesis,1983,p. 497 - 498
[8]Journal of the Chemical Society. Perkin transactions I,1980,p. 2812 - 2817
[9]Tetrahedron Letters,2007,vol. 48,p. 3379 - 3383
[10]Patent: US2003/229226,2003,A1 .Location in patent: Page 11
[11]Zeitschrift fur Naturforschung, B: Chemical Sciences,2010,vol. 65,p. 433 - 444
- 3
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
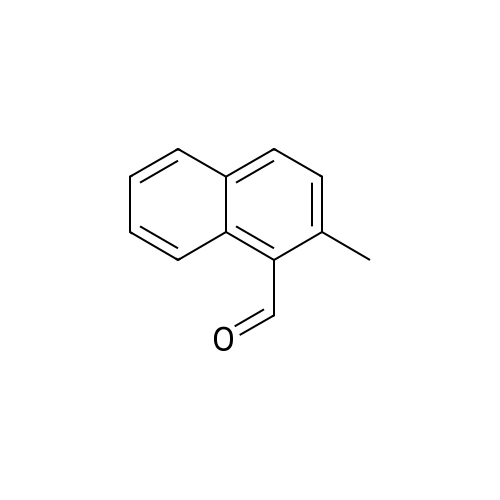 [ 35699-44-6 ]
[ 35699-44-6 ]

-
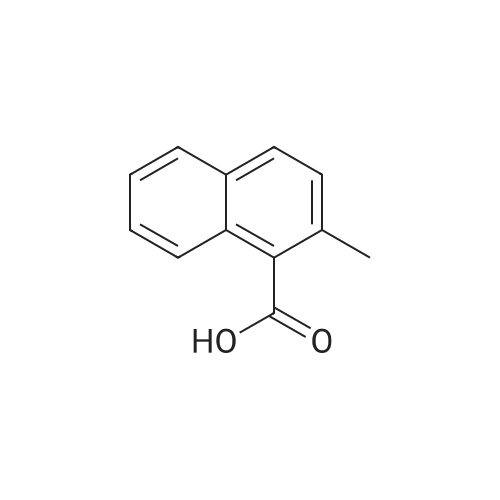 [ 1575-96-8 ]
[ 1575-96-8 ]

-
 [ 74661-65-7 ]
[ 74661-65-7 ]
- 4
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 35699-44-6 ]
[ 35699-44-6 ]

-
 [ 1575-96-8 ]
[ 1575-96-8 ]

-
 [ 126558-71-2 ]
[ 126558-71-2 ]
- 5
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 61172-29-0 ]
[ 61172-29-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
With N-Bromosuccinimide In tetrachloromethane Heating; |
|
- 6
-
 [ 219661-48-0 ]
[ 219661-48-0 ]

-
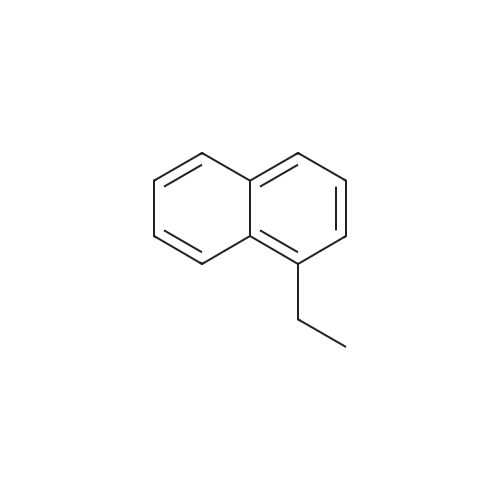 [ 1127-76-0 ]
[ 1127-76-0 ]

-
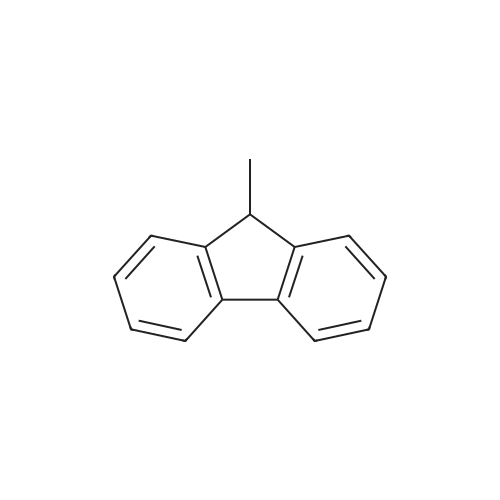 [ 2523-37-7 ]
[ 2523-37-7 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 91-57-6 ]
[ 91-57-6 ]
- 7
-
 [ 219661-48-0 ]
[ 219661-48-0 ]

-
 [ 1127-76-0 ]
[ 1127-76-0 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]

-
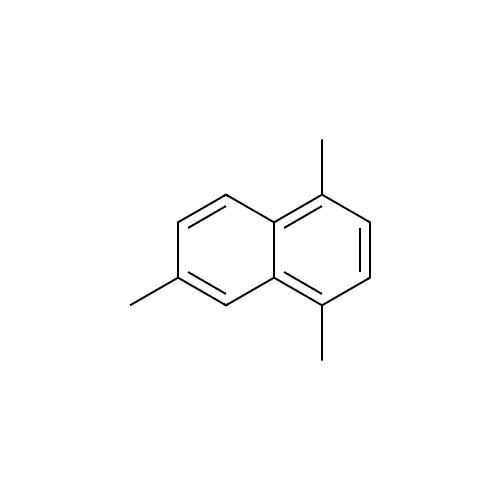 [ 2131-42-2 ]
[ 2131-42-2 ]

-
 [ 91-57-6 ]
[ 91-57-6 ]
- 8
-
 [ 219661-48-0 ]
[ 219661-48-0 ]

-
 [ 1127-76-0 ]
[ 1127-76-0 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]

-
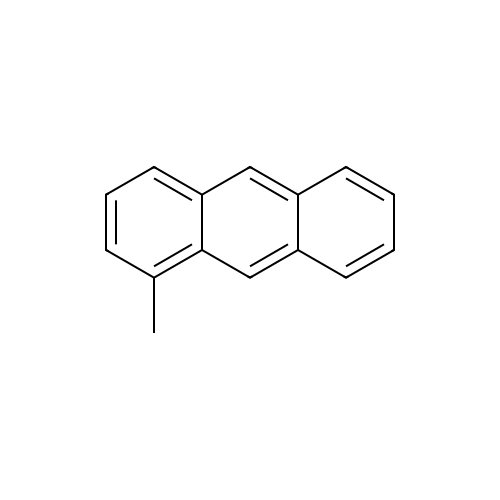 [ 610-48-0 ]
[ 610-48-0 ]

-
 [ 91-57-6 ]
[ 91-57-6 ]
- 9
-
 [ 219661-48-0 ]
[ 219661-48-0 ]

-
 [ 1127-76-0 ]
[ 1127-76-0 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]

-
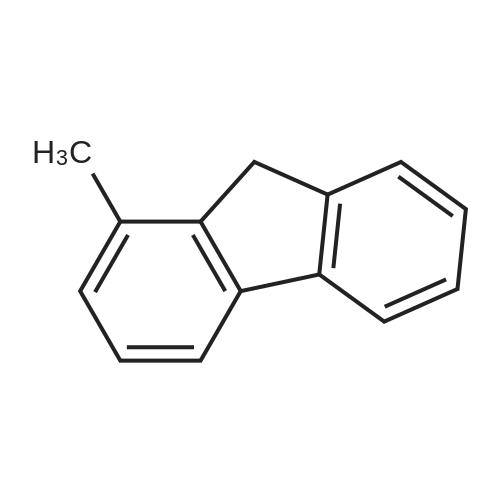 [ 1730-37-6 ]
[ 1730-37-6 ]

-
 [ 91-57-6 ]
[ 91-57-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With kieselguhr; nickel; benzene at 350 - 375℃; |
|
- 11
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 61172-29-0 ]
[ 61172-29-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With tetrachloromethane; N-Bromosuccinimide |
|
- 12
-
 [ 71-43-2 ]
[ 71-43-2 ]

-
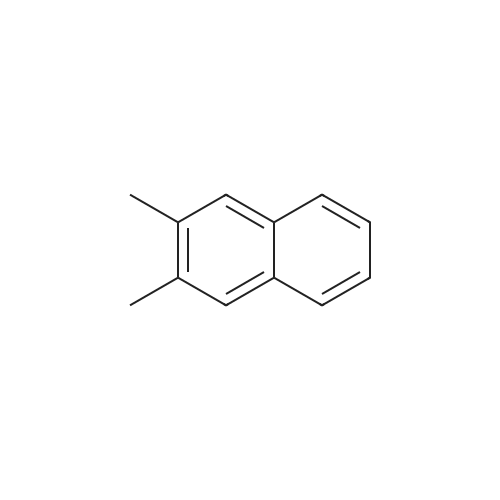 [ 581-40-8 ]
[ 581-40-8 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nickel kieselguhr at 350 - 375℃; |
|
- 13
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 1575-96-8 ]
[ 1575-96-8 ]
- 14
-
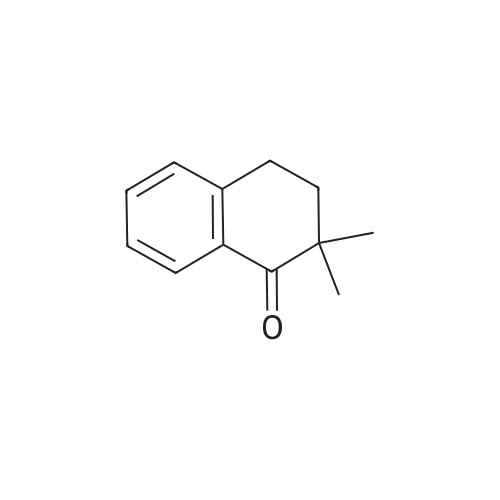 [ 2977-45-9 ]
[ 2977-45-9 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]
- 15
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
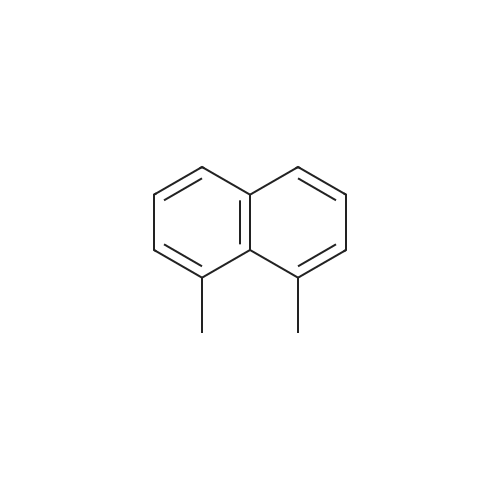 [ 569-41-5 ]
[ 569-41-5 ]

-
 [ 91-20-3 ]
[ 91-20-3 ]

-
 [ 581-40-8 ]
[ 581-40-8 ]

-
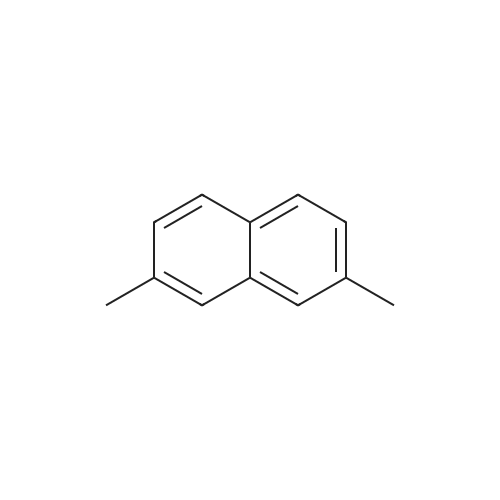 [ 582-16-1 ]
[ 582-16-1 ]

-
 [ 1127-76-0 ]
[ 1127-76-0 ]

-
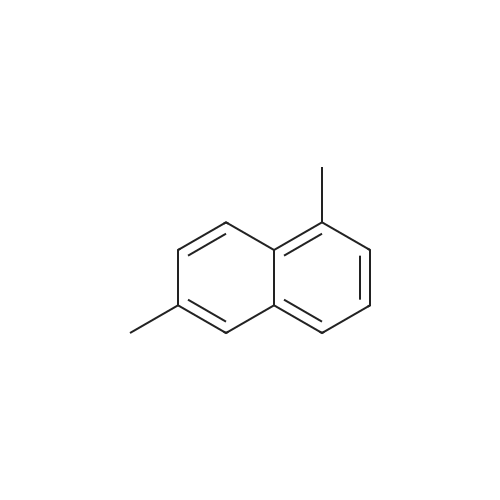 [ 575-43-9 ]
[ 575-43-9 ]

-
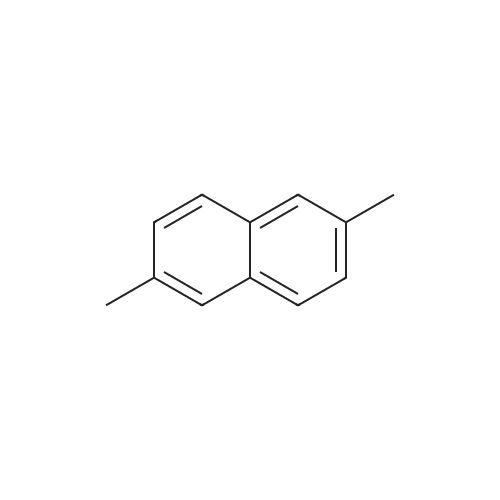 [ 581-42-0 ]
[ 581-42-0 ]

-
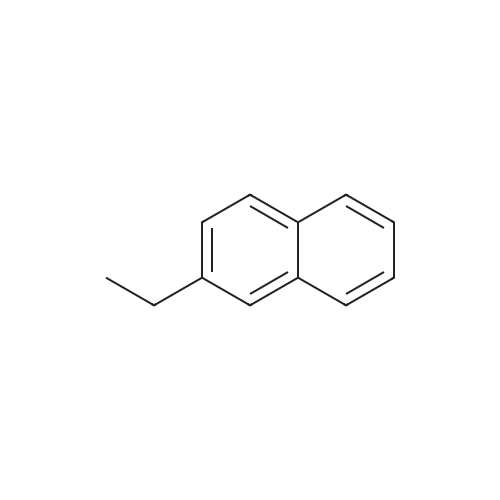 [ 939-27-5 ]
[ 939-27-5 ]

-
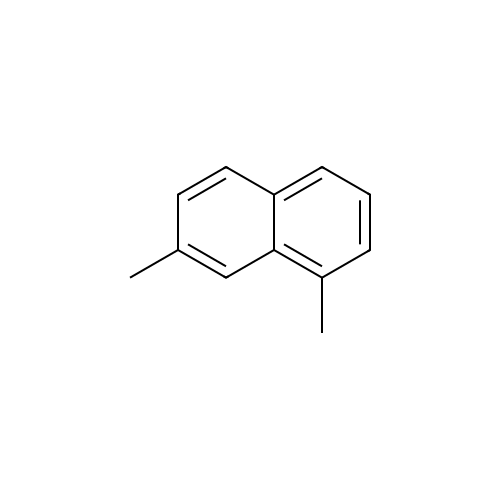 [ 575-37-1 ]
[ 575-37-1 ]

-
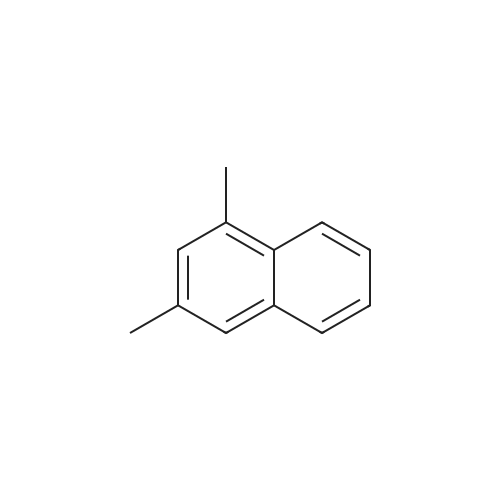 [ 575-41-7 ]
[ 575-41-7 ]

-
 [ 571-58-4 ]
[ 571-58-4 ]

-
 [ 571-61-9 ]
[ 571-61-9 ]

-
 [ 90-12-0 ]
[ 90-12-0 ]

-
 [ 91-57-6 ]
[ 91-57-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1.33%Chromat.; 2.56%Chromat.; 0.36%Chromat.; 1.43%Chromat.; 2.46%Chromat.; 1.56%Chromat.; 0.24%Chromat.; 1.05%Chromat.; 4.31%Chromat. |
With hydrogen;chromium corundum; at 20 - 500.6℃; under 59631 Torr;Purification / work up; |
Example 9 (Hydrodealkylation) A 70 g amount of Cr2O3/Al2O3 type catalyst produced by Sud-Chemie AG is charged into a tubular reactor. The reactor is heated gradually from ambient temperature to 932 F (500 C) to dry the catalyst while supplying hydrogen gas. Thereupon distillation product (Blend-B) obtained from Example 8 is supplied to the reactor at the rate of 70 g/hr and 1.0 hr-1 in WHSV, while supplying hydrogen gas at 0.98 scf/hr (0.028 m3/hr). The hydrodealkylation reaction is carried out at 933 F (500.6 C) and 1138 psig (7.95 MPa). The product is analyzed by GC and the results of hydrodealkylation are summarized in Table 8 below. |
| 4.75%Chromat.; 3.76%Chromat.; 0.44%Chromat.; 2.17%Chromat.; 3.53%Chromat.; 2.13%Chromat.; 0.34%Chromat.; 2.44%Chromat.; 9.48%Chromat. |
With hydrogen;cobalt oxide and molybdenum oxide on alumina; at 20 - 500℃; under 74482.4 Torr; |
Example 10 (Hydrodealkylation) A 70 g amount of CoO/MoO3/Al2O3 type catalyst produced by Akzo Chemicals Inc. is charged into a tubular reactor. The reactor is heated gradually from ambient temperature to 300F (148.9 C) with nitrogen flow at 5 scf/hr (0.142 m3/hr). Then the flow gas is switched to hydrogen at 2 scf/hr (0.057 m3/hr) and pressure is increased to 500 psig (3.55 MPa). Catalyst is contacted with an organic sulfide (Kerosene with 1.0% of Dimethyldisulfide) for sulfiding while supplying hydrogen gas and then temperature is raised to 650 F (343.3 C). Thereupon distillation product (Blend-B) obtained from Example 8 is fed to the reactor at the rate of 70 g/hr and 1.0 hr-1 in WHSV, while supplying hydrogen gas at 5scf/hr (0.028 m3/hr). Hydrodealkylation is carried out at 932 F (500 C) and 425 psig (9.93 Mpa) The product is analyzed by GC and the results of hydrodealkylation are summarized in Table 8 below. As shown in Table 8, both Cr2O3/Al2O3, and CoO/MoO3/Al2O3 type catalyst are effective to enrich DMN isomers from DMN lean feed. |
- 16
-
 [ 581-40-8 ]
[ 581-40-8 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 571-58-4 ]
[ 571-58-4 ]

-
 [ 91-20-3 ]
[ 91-20-3 ]

-
 [ 582-16-1 ]
[ 582-16-1 ]

-
 [ 1127-76-0 ]
[ 1127-76-0 ]

-
 [ 575-43-9 ]
[ 575-43-9 ]

-
 [ 581-42-0 ]
[ 581-42-0 ]

-
 [ 939-27-5 ]
[ 939-27-5 ]

-
 [ 575-37-1 ]
[ 575-37-1 ]

-
 [ 575-41-7 ]
[ 575-41-7 ]

-
 [ 569-41-5 ]
[ 569-41-5 ]

-
 [ 571-61-9 ]
[ 571-61-9 ]

-
 [ 90-12-0 ]
[ 90-12-0 ]

-
 [ 91-57-6 ]
[ 91-57-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 3.11%Chromat.; 4.58%Chromat.; 0.26%Chromat.; 2.92%Chromat.; 4.86%Chromat.; 0.57%Chromat.; 0.12%Chromat.; 0.39%Chromat.; 3.71%Chromat.; 15.68%Chromat. |
With hydrogen;chromium corundum; at 20 - 475℃; under 44929.5 Torr;Conversion of starting material; |
Example 4 (Hydrodealkylation) A part of Fraction-17 and Residue shown in Table 3 are mixed to prepare the feedstock(Blend-A) for hydrodealkylation. A 50 g amount of Cr2O3/Al2O3 type catalyst produced by Sud-Chemie AG is charged into a tubular reactor. The reactor is heated gradually from ambient temperature to 662F (350 C) to dry the catalyst while supplying hydrogen gas. Thereupon Blend-A is fed to the reactor at the rate of 50 g/hr and 1.0 hr-1 in WHSV, while supplying hydrogen gas at 1.25 cf/hr (0.034 m3/hr). Hydrodealkylation is carried out at 887F (475 C) and 854 psig (5.99 MPa). The product is analyzed by GC and the results of hydrodealkylation are summarized in Table 4 below. As shown in Table 4, Cr2O3/Al2O3 type catalyst is effective to enrich 2,6-DMN from 2,6-DMN lean feed. |
- 17
-
 [ 573-98-8 ]
[ 573-98-8 ]

-
 [ 34241-39-9 ]
[ 34241-39-9 ]

-
 [ 59882-98-3 ]
[ 59882-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With N-Bromosuccinimide; In tetrachloromethane; dichloromethane; |
(1) Preparation of 1,2-bis(bromomethyl)naphthalene In 300 ml of carbon tetrachloride, 10 g (64 mmole) of 1,2-dimethylnaphthalene and 46 g (260 mmole) of N-bromosuccinimide were suspended. To the obtained suspension, 1.7 g (260 mmole) of 2,2'-azobis(isobutyronitrile) was added and the mixture was vigorously stirred at 10C for 2 hours. After the reaction was completed, the reaction mixture was filtered and the residue was washed with 150 ml of dichloromethane. The filtrate and the washing were combined. The combined solution was treated by the column chromatography (silica gel / dichloromethane) without additional treatments and a white solid of 1,2-bis(bromomethyl)naphthalene was obtained in the quantitative amount. The 1H-NMR spectrum of the product was obtained and the result was as follows: 1H-NMR (CDCl3, TMS) delta: 4.74 (2H, s), 4.98 (2H, s), 7.3-8.1 (6H, m) |
- 18
-
 [ 13061-96-6 ]
[ 13061-96-6 ]

-
 [ 881-03-8 ]
[ 881-03-8 ]

-
 [ 573-98-8 ]
[ 573-98-8 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping




























