Alternatived Products of [ 61941-56-8 ]
Product Details of [ 61941-56-8 ]
| CAS No. : | 61941-56-8 |
MDL No. : | MFCD09842317 |
| Formula : |
C15H12NNaO3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
277.25
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 61941-56-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 61941-56-8 ]
- 1
-
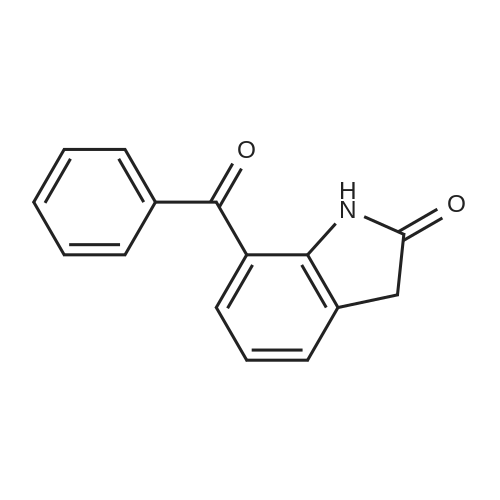 [ 51135-38-7 ]
[ 51135-38-7 ]

-
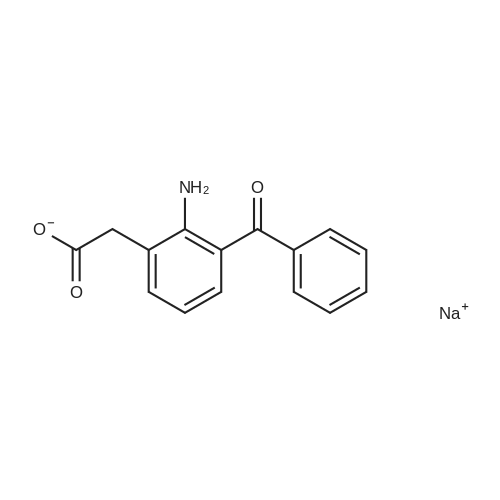 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
With sodium hydroxide In ethanol; toluene for 5h; Heating; |
|
| 80% |
With sodium hydroxide for 18h; Heating; |
|
- 2
-
 [ 61941-56-8 ]
[ 61941-56-8 ]

-
 [ 126849-29-4 ]
[ 126849-29-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 56% |
With hydrogen In water at 50 - 60℃; for 32h; |
|
- 3
-
 [ 61941-56-8 ]
[ 61941-56-8 ]

-
 [ 79588-33-3 ]
[ 79588-33-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 53% |
With hydrogen In water Ambient temperature; |
|
- 4
-
 [ 61085-33-4 ]
[ 61085-33-4 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 94 percent / Raney nickel / tetrahydrofuran / 0.17 h
2: 80 percent / 3N aq. NaOH / 18 h / Heating |
|
- 5
-
(2-Amino-3-benzoyl-phenyl)-methylsulfanyl-acetic acid ethyl ester
[ No CAS ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: 3N aq. HCl / CH2Cl2 / 2 h / Heating
2: 94 percent / Raney nickel / tetrahydrofuran / 0.17 h
3: 80 percent / 3N aq. NaOH / 18 h / Heating |
|
- 6
-
C18H20NO3S(1+)*Cl(1-)
[ No CAS ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: Et3N / CH2Cl2 / -70 - 20 °C
2: 3N aq. HCl / CH2Cl2 / 2 h / Heating
3: 94 percent / Raney nickel / tetrahydrofuran / 0.17 h
4: 80 percent / 3N aq. NaOH / 18 h / Heating |
|
- 7
-
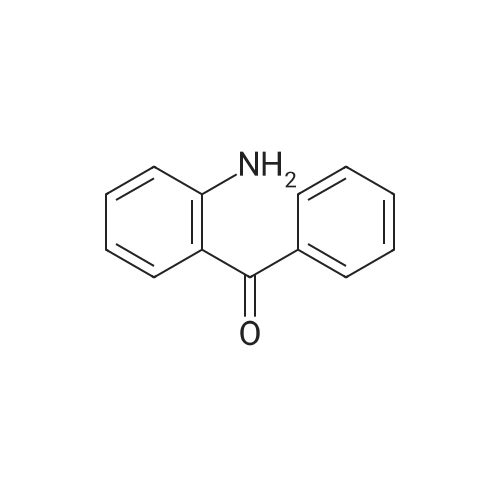 [ 2835-77-0 ]
[ 2835-77-0 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: tert-butyl hypochlorite / CH2Cl2 / 1 h / -70 °C
2: Et3N / CH2Cl2 / -70 - 20 °C
3: 3N aq. HCl / CH2Cl2 / 2 h / Heating
4: 94 percent / Raney nickel / tetrahydrofuran / 0.17 h
5: 80 percent / 3N aq. NaOH / 18 h / Heating |
|
- 8
-
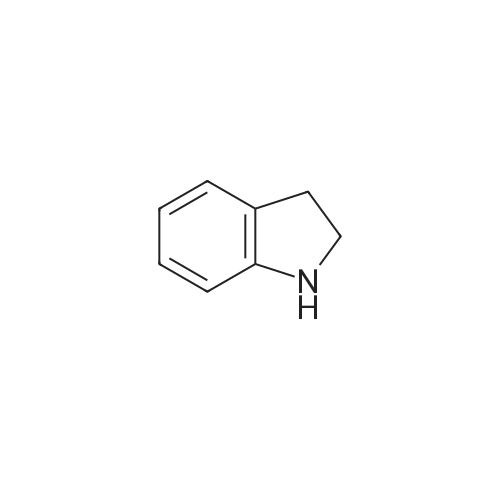 [ 496-15-1 ]
[ 496-15-1 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: 80 percent / BCl3, AlCl3 / toluene / 16 h / Heating
2: MnO2 / CH2Cl2 / 18 h / Heating
3: N-chlorosuccinimide / CH2Cl2 / 1 h / 15 - 20 °C
4: 70percent aq. H3PO4 / 2-methoxy-ethanol / 8 h / Heating
5: 86 percent / 50percent aq. sodium hydroxide / toluene; ethanol / 5 h / Heating |
|
- 9
-
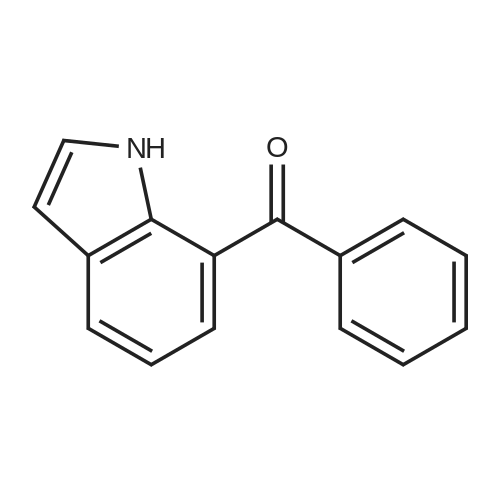 [ 70803-96-2 ]
[ 70803-96-2 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: N-chlorosuccinimide / CH2Cl2 / 1 h / 15 - 20 °C
2: 70percent aq. H3PO4 / 2-methoxy-ethanol / 8 h / Heating
3: 86 percent / 50percent aq. sodium hydroxide / toluene; ethanol / 5 h / Heating |
|
- 10
-
 [ 33244-57-4 ]
[ 33244-57-4 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: MnO2 / CH2Cl2 / 18 h / Heating
2: N-chlorosuccinimide / CH2Cl2 / 1 h / 15 - 20 °C
3: 70percent aq. H3PO4 / 2-methoxy-ethanol / 8 h / Heating
4: 86 percent / 50percent aq. sodium hydroxide / toluene; ethanol / 5 h / Heating |
|
- 11
-
 [ 76049-81-5 ]
[ 76049-81-5 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 70percent aq. H3PO4 / 2-methoxy-ethanol / 8 h / Heating
2: 86 percent / 50percent aq. sodium hydroxide / toluene; ethanol / 5 h / Heating |
|
- 12
-
 [ 100-47-0 ]
[ 100-47-0 ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: 80 percent / BCl3, AlCl3 / toluene / 16 h / Heating
2: MnO2 / CH2Cl2 / 18 h / Heating
3: N-chlorosuccinimide / CH2Cl2 / 1 h / 15 - 20 °C
4: 70percent aq. H3PO4 / 2-methoxy-ethanol / 8 h / Heating
5: 86 percent / 50percent aq. sodium hydroxide / toluene; ethanol / 5 h / Heating |
|
| Yield | Reaction Conditions | Operation in experiment |
|
|
1. Sodium 3-(4-bromobenzoyl)-2-aminophenyl-acetate (hereinafter referred to as Compound [I]) Sodium 3-(4-chlorobenzoyl)-2-aminophenyl-acetate (hereinafter referred to as Compound [II]) Sodium 3-benzoyl-2-aminophenylacetate (hereinafter referred to as Compound [III]) |
- 14
-
zinc(II) sulfate heptahydrate
[ No CAS ]

-
 [ 61941-56-8 ]
[ 61941-56-8 ]

-
2-amino-3-benzoylbenzeneacetic acid, zinc salt
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water |
71 2-Amino-3-benzoylbenzeneacetic acid, zinc salt
EXAMPLE 71 2-Amino-3-benzoylbenzeneacetic acid, zinc salt A solution of 6.36 g (0.021 mole) of 2-amino-3-benzoylbenzeneacetic acid, sodium salt in 100 ml of water was treated dropwise with a solution of 2.94 g of zinc sulfate heptahydrate (0.01 mole) in 100 ml of water. A precipitate instantly developed. The mixture was stirred for 10 minutes and the precipitate filtered off to give 4.4 g of product, recrystallized from toluene-petroleum ether, m.p. 95°-140° C. Analysis: Calculated for C30 H24 N2 O6 Zn: C, 62.79; H, 4.22; N, 4.88 Found: C, 62.78; H, 4.17; N, 4.84. |
- 15
-
 [ 61941-56-8 ]
[ 61941-56-8 ]

-
 [ 74-88-4 ]
[ 74-88-4 ]

-
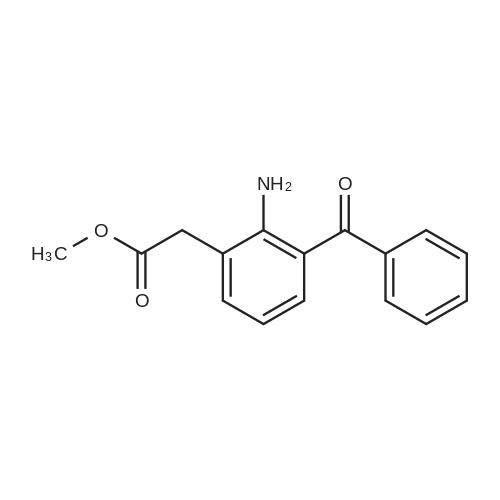 [ 61941-58-0 ]
[ 61941-58-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 3.5 gms. (90%) |
In <i>N</i>-methyl-acetamide |
10 EXAMPLE 10
EXAMPLE 10 Methyl 2-amino-3-benzoylphenylacetate. A solution of 4.0 g. (0.014 mole) of the sodium salt of 2-amino-3-benzoylphenylacetic acid in 100 ml. of dry dimethylformamide was treated with 8.0 g. (0.057 mole) of methyl iodide. After stirring for two hours the solution was poured into water and the aqueous solution extracted several times with ethyl ether. The combined extracts were washed with water, dried over sodium sulfate and concentrated under vacuum to a yellow oil. The oil was crystallized from a chilled methanol-water solution to give 3.5 gms. (90%) of a yellow solid which melted at 52°-54° C. Analysis: Calc'd for C16 H15 NO3: C,71.36; H,5.61; N,5.20 Found: C,71.51; H,5.63; N,5.27. |
- 16
-
 [ 61941-56-8 ]
[ 61941-56-8 ]

-
 [ 75-03-6 ]
[ 75-03-6 ]

-
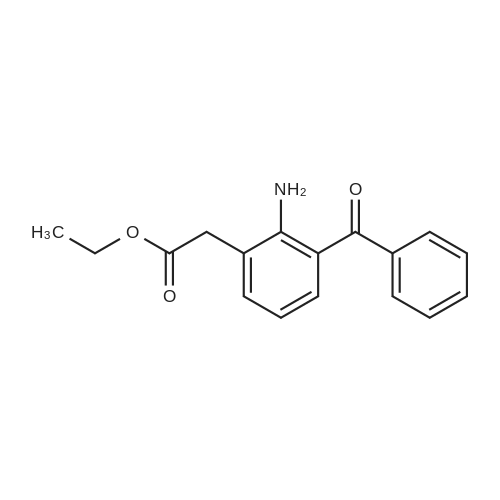 [ 61941-57-9 ]
[ 61941-57-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1.7 gms. (61.0%) |
In <i>N</i>-methyl-acetamide |
9 EXAMPLE 9
EXAMPLE 9 Ethyl 2-amino-3-benzoylphenylacetate. A solution of 2.5 g. (0.009 mole) of the sodium salt of 2-amino-3-benzoylphenylacetic acid in 25 ml. of dry dimethylformamide was treated with 5.0 g. (0.035 mole) of ethyl iodide. The mixture was stirred two hours at room temperature using a magnetic stirrer. The mixture was diluted with water and the aqueous solution extracted several times with ethyl ether. The combined extracts were washed with water, dried over sodium sulfate and concentrated under vacuum to a yellow solid. The solid was recrystallized from absolute ethanol to give 1.7 gms. (61.0%) of yellow needles which melted at 77°-78° C. Analysis: Calc'd for C17 H17 NO3: C,72.07; H,6.05; N,4.94 Found: C,72.33; H,5.83; N,5.07 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping












