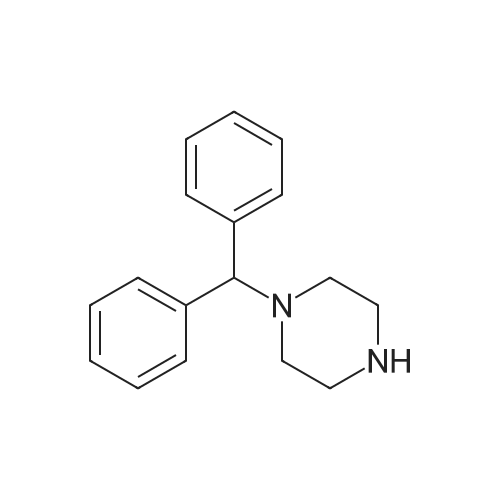
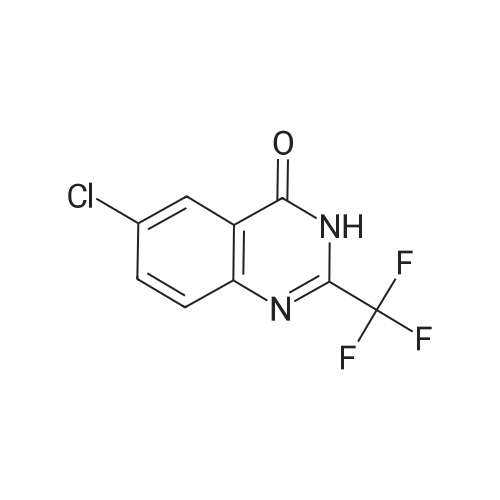
| 89% |
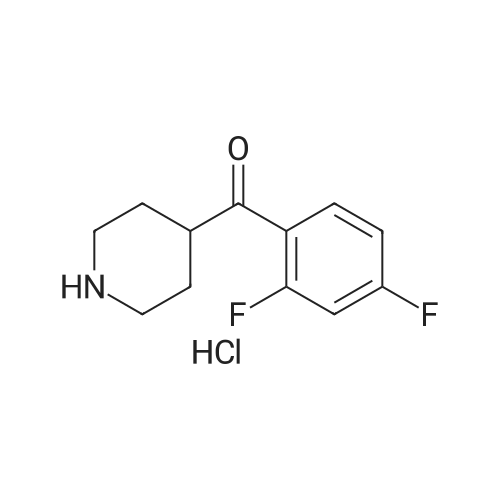
With sodium carbonate; potassium iodide In N,N-dimethyl-formamide at 80℃; |
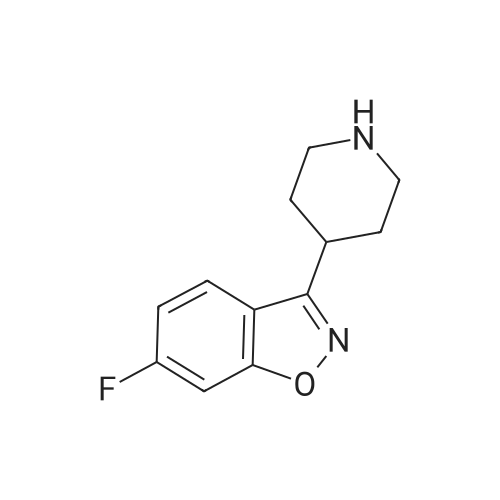
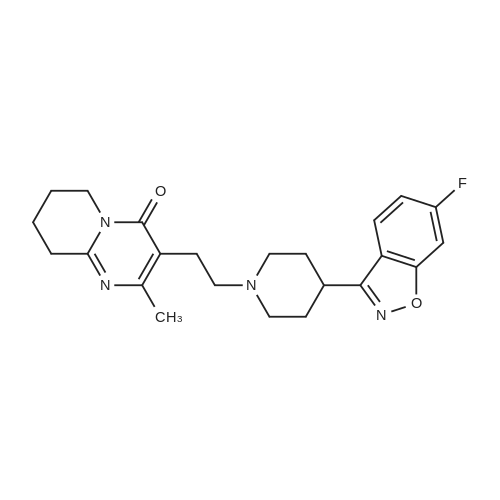
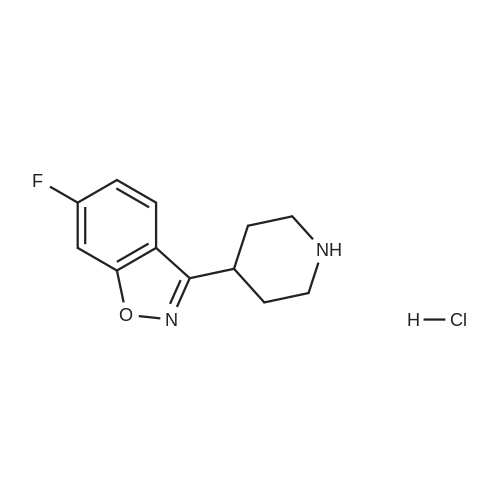
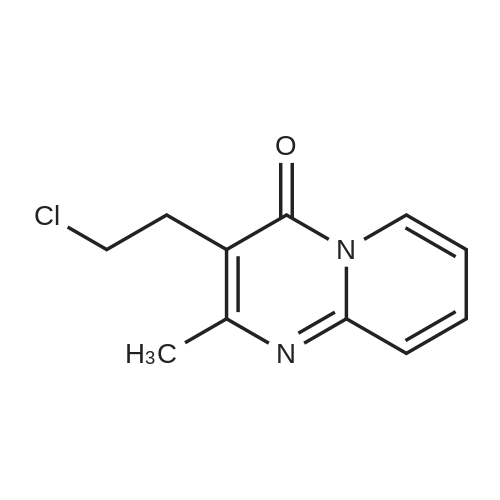
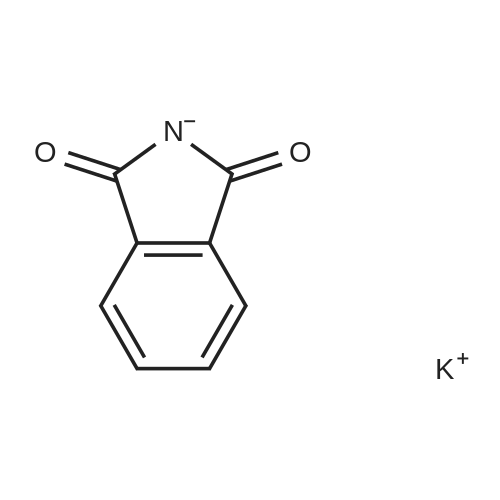
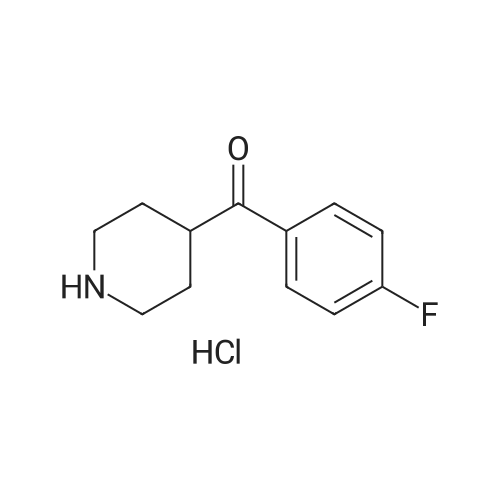
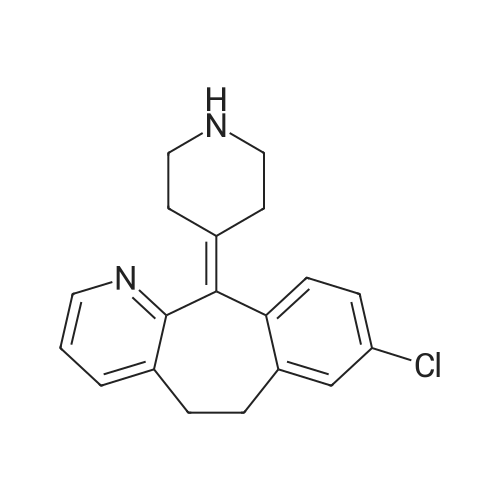
9.2 Step 2: Preparation of 3-(2-(4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
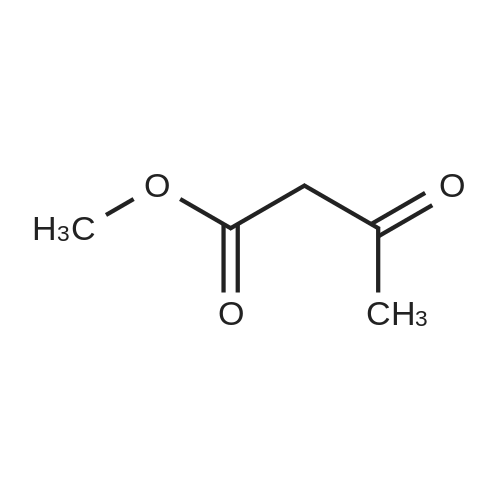
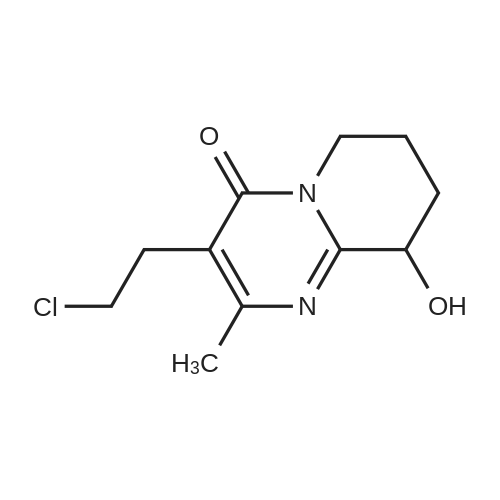
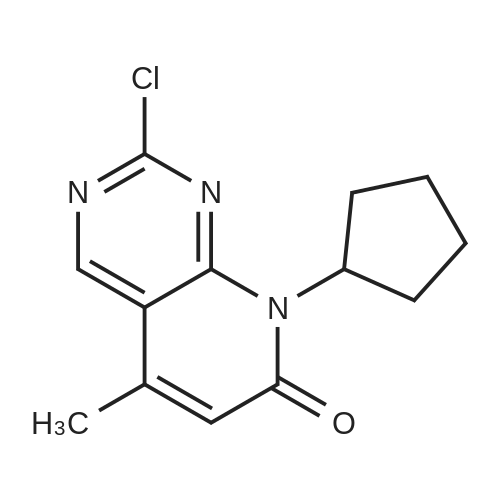
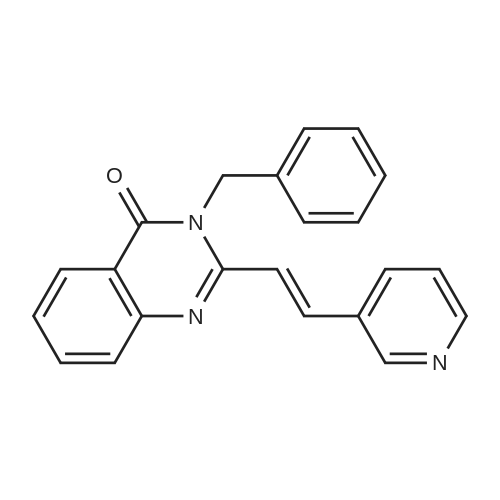
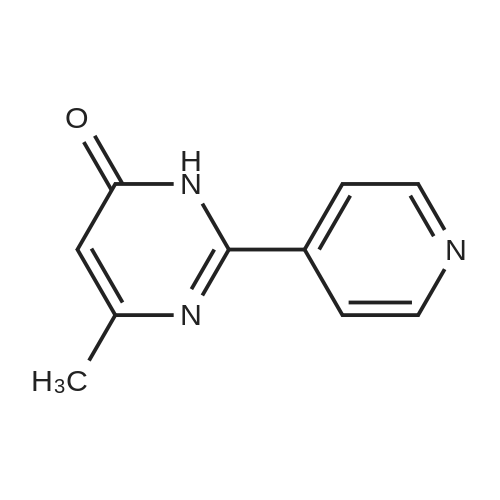
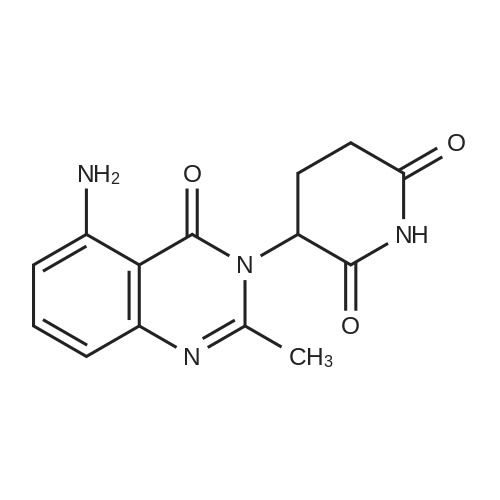
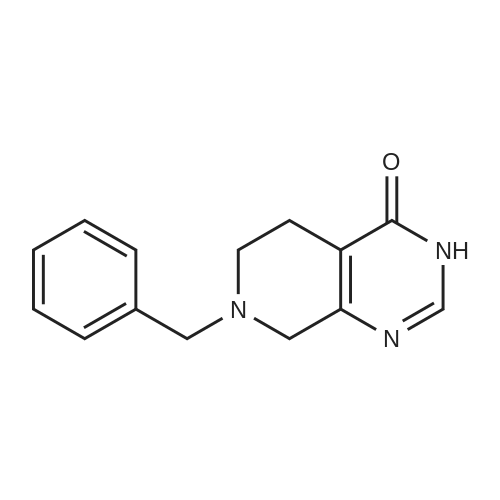
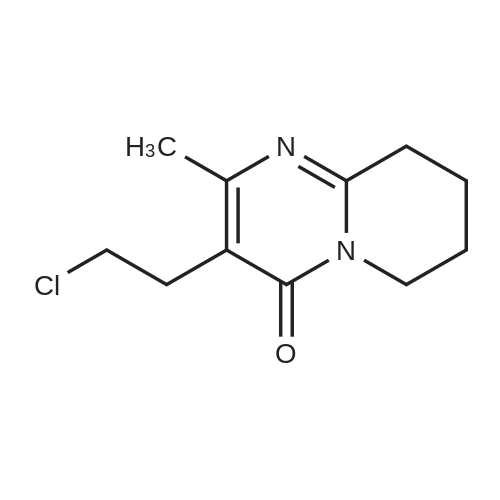
After the compound (120 mg, 0.313 mmol) obtained in the step 1 of Example 9 was dissolved in 2 mL ofN,N-dimethylformamide, sodium carbonate (99.43 mg, 0.938 mmol), potassium iodide (51.91 mg, 0.313 mmol), 3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one (77.74 mg, 0.344 mmol) were sequentially added, and the resultant was then heated to 80°C. After the completion of the reaction, extraction was performed twice with brine and ethyl acetate. The collected organic layer was dried over anhydrous sodium sulfate and then concentrated. The residue was purified by silica gel column chromatography to obtain a title compound (140 mg, yield of 89%). 1H NMR (300 MHz, MeOD): δ 8.49 (dd, J=4.88, 1.22 Hz, 1H), 7.87 (d, J=7.63 Hz, 1H), 7.50-7.44 (m, 1H), 7.38 (d, J=2.14 Hz, 1H), 7.35-7.23 (m, 2H), 4.92-4.78 (m, 2H), 3.65-3.28 (m, 9H), 2.96-2.43 (m, 16H) |
| 89% |
With sodium carbonate; potassium iodide In N,N-dimethyl-formamide at 80℃; |
9.2 Step 2: Preparation of 3-(2-(4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
After the compound (120 mg, 0.313 mmol) obtained in the step 1 of Example 9 was dissolved in 2 mL ofN,N-dimethylformamide, sodium carbonate (99.43 mg, 0.938 mmol), potassium iodide (51.91 mg, 0.313 mmol), 3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one (77.74 mg, 0.344 mmol) were sequentially added, and the resultant was then heated to 80°C. After the completion of the reaction, extraction was performed twice with brine and ethyl acetate. The collected organic layer was dried over anhydrous sodium sulfate and then concentrated. The residue was purified by silica gel column chromatography to obtain a title compound (140 mg, yield of 89%). 1H NMR (300 MHz, MeOD): δ 8.49 (dd, J=4.88, 1.22 Hz, 1H), 7.87 (d, J=7.63 Hz, 1H), 7.50-7.44 (m, 1H), 7.38 (d, J=2.14 Hz, 1H), 7.35-7.23 (m, 2H), 4.92-4.78 (m, 2H), 3.65-3.28 (m, 9H), 2.96-2.43 (m, 16H) |
|
|
9.2 Step 2:
Step 2: Preparation of 3-(2-(4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one After the compound (120 mg, 0.313 mmol) obtained in the step 1 of Example 9 was dissolved in 2 mL ofN,N-dimethylformamide, sodium carbonate (99.43 mg, 0.938 mmol), potassium iodide (51.91 mg, 0.313 mmol), 3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one (77.74 mg, 0.344 mmol) were sequentially added, and the resultant was then heated to 80°C. After the completion of the reaction, extraction was performed twice with brine and ethyl acetate. The collected organic layer was dried over anhydrous sodium sulfate and then concentrated. The residue was purified by silica gel column chromatography to obtain a title compound (140 mg, yield of 89%). 1H NMR (300 MHz, MeOD): δ 8.49 (dd, J=4.88, 1.22 Hz, 1H), 7.87 (d, J=7.63 Hz, 1H), 7.50-7.44 (m, 1H), 7.38 (d, J=2.14 Hz, 1H), 7.35-7.23 (m, 2H), 4.92-4.78 (m, 2H), 3.65-3.28 (m, 9H), 2.96-2.43 (m, 16H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping