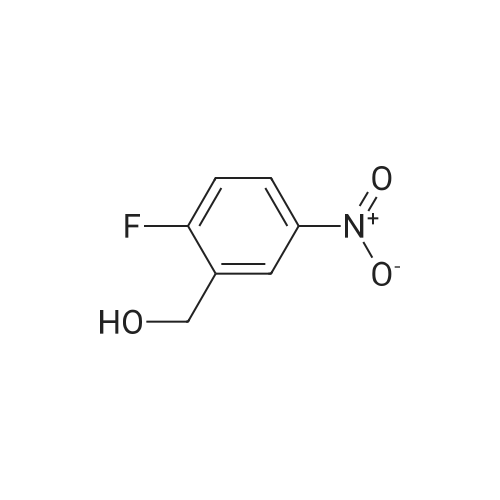
| 4.5 g |
With diethylamino-sulfur trifluoride; In dichloromethane; at -78℃;Inert atmosphere; |
To a solution of Compound 2 (6.2 g, 36.3 mmol) in anhydrous DCM (80 mL) was added DAST (11.7 g, 34.3 mmol) drop-wise at -78 C under N2. The reaction mixture was stirred at rt for 2 h, and poured into a beaker containing 30 g of ice, decomposing any unreacted DAST. Mixture was extracted twice with 45 mL portions of DCM. The combined organic layer was washed with 50 mL of water, and dried over anhydrous magnesium sulfate. Evaporation to dryness under reduced pressure gives crude product which was purified by silica gel chromatography (eluted with PE: EA=froml00: 1 to 50: 1) to afford Compound 3. (4.5 g, yield: 71%) |
| 3.9 g |
With diethylamino-sulfur trifluoride; In dichloromethane; at -30 - 10℃; |
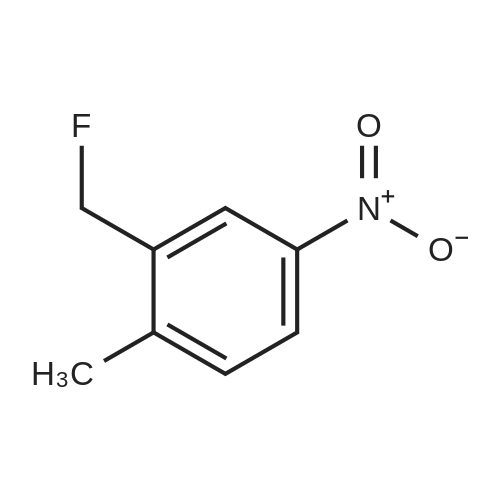
Compound 127 (2-fluoro-5-nitro-phenyl)methanol (4.3 g, 25.1 mmol) was dissolved in dichloro- methane (50 mL). Diethylaminosulfur trifluoride (4.5 g, 27.9 mmol) was added drop wise to the mixture at -30C. The mixture was stirred at 10 C for 4 hours. Methanol (10 mL) was added to the mixture and the mixture was further stirred at 10C for 30 minutes. The mixture was washed with brine (30mL) and the aqueous layer was extracted with CH2C12 (2 x 30 mL). The combined organic layers were dried over Na2S04 and concentrated in vacuo, resulting in l-fluoro-2-(fluoromethyl)-4-nitro- benzene (3.9 g). A mixture of l-fluoro-2-(fluoromethyl)-4-nitro-benzene (3.1 g, 17.9 mmol), iron (4.0 g, 71.6 mmol) and methanol (30 mL) was stirred at 65 for 8 hours. The mixture was filtrated and the filtrate was concentrated in vacuo, resulting in 4-fluoro-3-(fluoromethyl)aniline (1.5 g). 3-(chlorosulfonyl)benzoyl chloride (300 mg, 1.2 mmol) and triethylamine (150 mg, 1.5 mmol) were dissolved in dichloromethane (20 mL). 4-fluoro-3-(fluoromethyl)aniline (175 mg, 1.22 mmol) was added to the mixture at 0 C. The mixture was stirred at 10C for 30 minutes. The mixture was used to the next step without further purification.Triethylamine (152 mg, 1.5 mmol) and 3-methyl-3-oxetanamine (131 mg. 1.5 mmol) were added to the above obtained reaction mixture at 0 C. The mixture was stirred at 20 C for 1 hour. The solvent was removed in vacuo and the obtained residue was purified by reversed phase high performance liquid chromatography (Column: Gemini 250*20mm*5um.. A: H2O+0.1%TFA B: MeCN. 27% to 57% B in A). The product fractions were collected and the organic solvent was removed in vacuo. The fraction was neutralized by saturated NaHC03. The mixture was extracted with dichloromethane (3 x 20 mL) and the combined organic layer was dried over Na2S04 and concentrated in vacuo, resulting in compound 127 (91.1 mg). Method A; Rt: 4.95 min. m/z : 397.3 (M+H)+ Exact mass: 396.1. 1H NMR (400 MHz, DMSO-d6) delta ppm 1.41 (s, 3 H) 4.14 (d, J=6.3 Hz, 2 H) 4.56 (d, J=6.3 Hz, 2 H) 5.52 (d, J=48 Hz, 2 H) 7.31 (t, J=9.4 Hz, 1 H) 7.72 - 7.89 (m, 2 H) 7.92-7.97 (m, 1 H) 8.03 (d, J=8.0 Hz, 1 H) 8.23 (d, J=7.8 Hz, 1 H) 8.39 (s, 1 H) 8.55 (s, 1 H) 10.67 (s, 1 H). |
| 3.9 g |
With diethylamino-sulfur trifluoride; In dichloromethane; at -30 - 10℃; for 4.0h; |
Compound 127 (2-fluoro-5-nitro-phenyl)methanol (4.3 g, 25.1 mmol) was dissolved in dichloro- methane (50 mL). Diethylaminosulfur trifluoride (4.5 g, 27.9 mmol) was added drop wise to the mixture at -30C. The mixture was stirred at 10 C for 4 hours. Methanol (10 mL) was added to the mixture and the mixture was further stirred at 10C for 30 minutes. The mixture was washed with brine (30mL) and the aqueous layer was extracted with CH2C12 (2 x 30 mL). The combined organic layers were dried over Na2S04 and concentrated in vacuo, resulting in l-fluoro-2-(fluoromethyl)-4-nitro- benzene (3.9 g). A mixture of l-fluoro-2-(fluoromethyl)-4-nitro-benzene (3.1 g, 17.9 mmol), iron (4.0 g, 71.6 mmol) and methanol (30 mL) was stirred at 65 for 8 hours. The mixture was filtrated and the filtrate was concentrated in vacuo, resulting in 4-fluoro-3-(fluoromethyl)aniline (1.5 g). 3-(chlorosulfonyl)benzoyl chloride (300 mg, 1.2 mmol) and triethylamine (150 mg, 1.5 mmol) were dissolved in dichloromethane (20 mL). 4-fluoro-3-(fluoromethyl)aniline (175 mg, 1.22 mmol) was added to the mixture at 0 C. The mixture was stirred at 10C for 30 minutes. The mixture was used to the next step without further purification.Triethylamine (152 mg, 1.5 mmol) and 3-methyl-3-oxetanamine (131 mg. 1.5 mmol) were added to the above obtained reaction mixture at 0 C. The mixture was stirred at 20 C for 1 hour. The solvent was removed in vacuo and the obtained residue was purified by reversed phase high performance liquid chromatography (Column: Gemini 250*20mm*5um.. A: H2O+0.1%TFA B: MeCN. 27% to 57% B in A). The product fractions were collected and the organic solvent was removed in vacuo. The fraction was neutralized by saturated NaHC03. The mixture was extracted with dichloromethane (3 x 20 mL) and the combined organic layer was dried over Na2S04 and concentrated in vacuo, resulting in compound 127 (91.1 mg). Method A; Rt: 4.95 min. m/z : 397.3 (M+H)+ Exact mass: 396.1. 1H NMR (400 MHz, DMSO-d6) delta ppm 1.41 (s, 3 H) 4.14 (d, J=6.3 Hz, 2 H) 4.56 (d, J=6.3 Hz, 2 H) 5.52 (d, J=48 Hz, 2 H) 7.31 (t, J=9.4 Hz, 1 H) 7.72 - 7.89 (m, 2 H) 7.92-7.97 (m, 1 H) 8.03 (d, J=8.0 Hz, 1 H) 8.23 (d, J=7.8 Hz, 1 H) 8.39 (s, 1 H) 8.55 (s, 1 H) 10.67 (s, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping