| 70% |
|
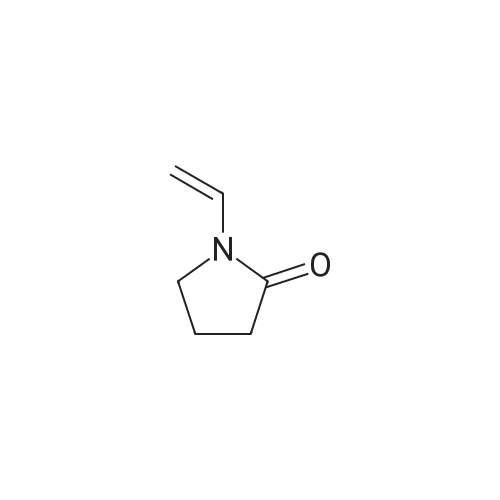
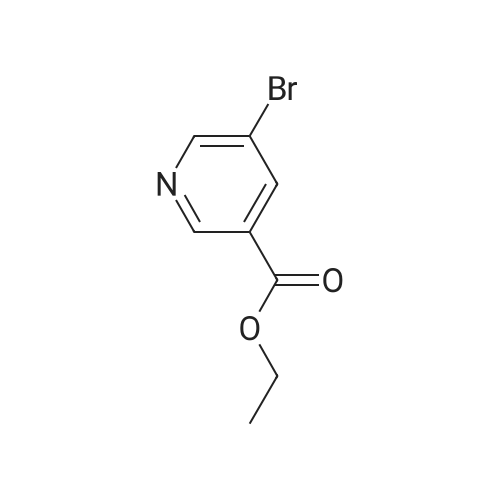
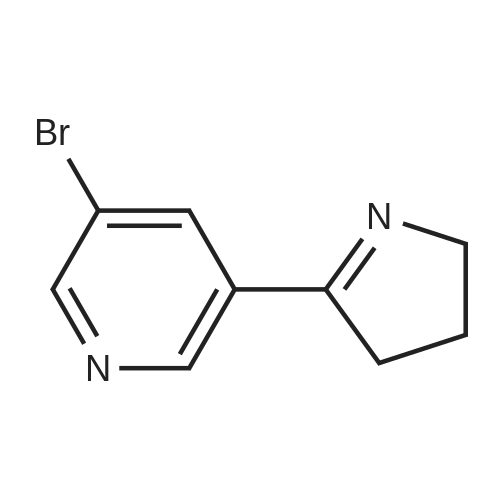
Sodium hydride (3.44 g, 86 mmol, 60% dispersion in oil) in a three-neck flask was washed with three 20 mE portions of hexane. The flask was fitted with a reflux condenser, flushed with argon, and charged with THF (70 mE). A solution of 1 (10 g, 66.1 mmol) and 1-vinyl-2- pyrrolidinone (7.89 g, 71 mmol) in THF (15 mE) was added in one portion. The mixture was stirred and refluxed for 1 h and then cooled to room temperature. A solution of concentrated HC1 (12 mE) in water (18 mE) was added, and the THF was removed on a rotary evaporator. Additional concentrated HC1 (18 mE) and water (36 mE) were added, and the mixture was heated at reflux overnight. In an ice-cooled bath, the solution was made basic with concentrated aqueous NaOH, which resulted in precipitation of the crude product, and then it was extracted with CH2C12 (2x75 mE). The combined CH2C12 layer was washed with water, brine, dried over anhydrous Mg504 and concentrated under diminished pressure. The residue was purified by chromatography on a silica gel colunm (15x5 cm) eluting with 19:1 CH2C12- acetone afforded 2 as a pale yellow solid: yield 6.87 g (70%); ?H NMR (400 MHz, CDC13) oe 2.00-2.08 (m, 2H), 2.86-2.92 (m, 2H), 4.02-4.08 (m, 2H), 8.31 (t, 1H, J=1.6 Hz), 8.67 (d, 1H, J=2.0 Hz) and 8.83 (d, 1H, J=1.6 Hz); ?3C NMR (100 MHz, CDC13) oe 22.6, 34.9, 61.8, 121.0, 131.7, 137.2, 147.1, 152.2 and 169.9. |
| 53% |
|
To a stirred solution containing ethyl 5-bromonicotinate (3.49 g, 15.2 mmol) , N-vinylpyrrolidinone (2.03 g, 18.2 mmol, 1.2 equiv) and dry THF (5.1 mL) under N2, NaH (0.511 g, 21.3 mmol, 1.4 equiv) in 14.2 mL dry THF was added. The reaction was left to stir for 10 - 15 min until the exothermic reaction was complete and then brought to reflux conditions. After 1 h, the reaction mixture was allowed to cool to room temperature and 4.47 mL of 4.7 M HCl (2 equiv) was added with stirring. The solvent was removed en vacuo and 10.7 mL of 4.7 M HCl (3.33 equiv) was added to the residue. The solution was brought to reflux conditions and left to stir for 18 h. After cooling the reaction mixture to room temperature, 50% NaOH was added portion-wise, with vigorous stirring, to make the solution basic and a thick precipitate formed. The basic solution was extracted twice with dichloromethane. The organic fractions were combined and solvent removed in vacuo. Crude material (1.8O g, 8.0 mmol, 53% crude yield) was used for further reactions. A fraction of the material was purified for characterization (flash chromatography on Si gel, 2% MeOH/CH2C12, Rf = 0.21) . 1H NMR (300 MHz,CDC13) 6 ppm, 8.82 (d, IH), 8.65 Cd, 1H), 8.29 (t, IH), 4.04 (tt, 2H), 2.88 (m, IH), 2.03 {m, 2H). LCMS (ESI+) , m/z [M+H] + = 225.14. |
|
|
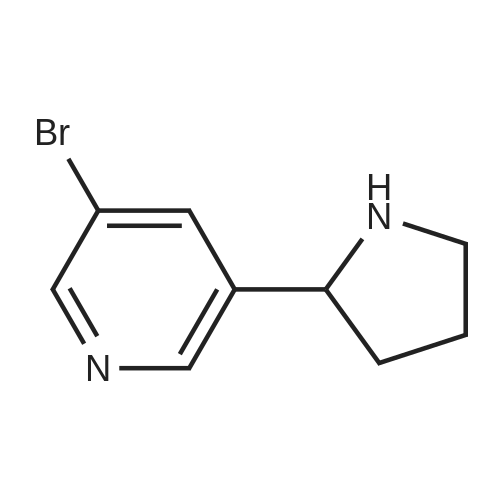
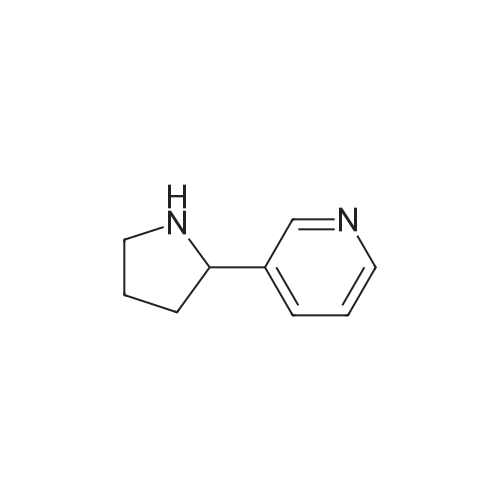
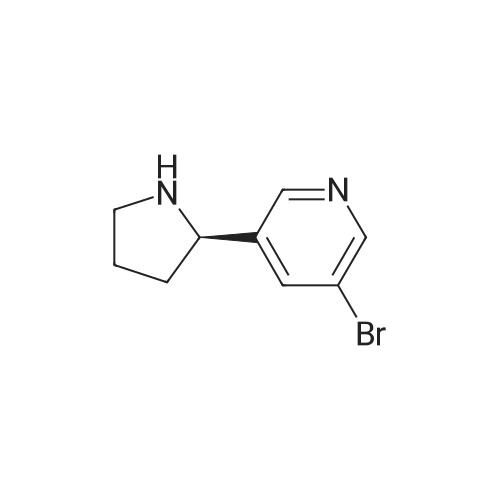
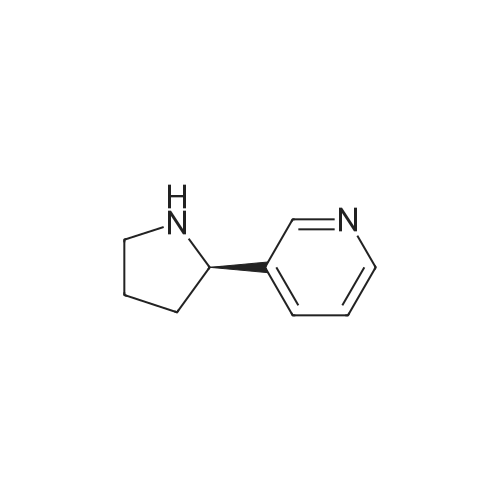
As exemplified for the nicotine hapten shown at the upper left of the FIG. 18, (S)-nornicotine (6) was synthesized, as shown in FIG. 19, starting from 5-bromonicotinic acid (1) (Scheme 1), which upon esterification gave compound 2. Bromo ester 2 was condensed with N-vinyl pyrrolidinone, followed by in situ acid catalyzed hydrolysis, decarboxylation and base-promoted cyclization to afford compound 3. Imine 3 upon reduction with sodium borohydride provided racemic 5-bromonornicotine (4). This racemic mixture was resolved with alpha-methoxy-alpha-trifluoromethyl phenyl acetic acid (MTPA) as the corresponding MTPA salts ((R)-5-bromonornicotine MTPA salt (5a) and (S)-5-bromonornicotine MTPA salt (5b)). The (S)-bromonornicotine MTPA salt (5b) upon reductive debromination with hydrogen and palladium catalyst afforded (S)-nornicotine (6, Scheme 1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping