Alternatived Products of [ 683242-75-3 ]
Product Details of [ 683242-75-3 ]
| CAS No. : | 683242-75-3 |
MDL No. : | MFCD22561321 |
| Formula : |
C9H7ClN2O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
210.62
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 683242-75-3 ]
| Signal Word: | |
Class: | N/A |
| Precautionary Statements: | |
UN#: | N/A |
| Hazard Statements: | |
Packing Group: | N/A |
Application In Synthesis of [ 683242-75-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 683242-75-3 ]
- 1
-
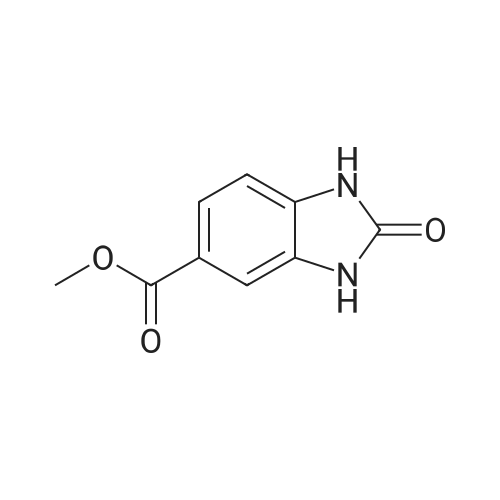 [ 106429-57-6 ]
[ 106429-57-6 ]

-
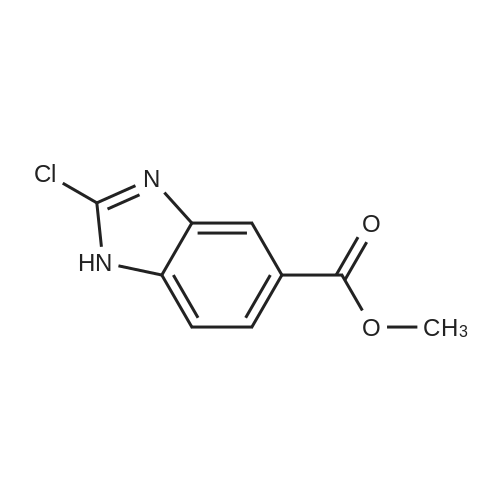 [ 683242-75-3 ]
[ 683242-75-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 84% |
|
Methyl 2-oxo-2,3-dihydro-1H-benzimidazole-6-carboxylate (43 g, 0.22 mol) was charged into Phosphorus oxychloride (286 ml). Dry hydrogen chloride was bubbled through the boiling reaction mass for 12 hours. After cooling reaction mass was poured in mixture of ice and water (2 kg). Precipitate was filtered out. Filtrate was diluted with water (1.25 l) and ammonia solution (800 ml). After that pH was adjusted to 5.6 with ammonia solution. Precipitate was filtered and rinsed with water. Yield 39.5 g (84%). |
| 36% |
With trichlorophosphate; at 90℃; for 1h; |
Methyl 2-oxo-2,3-dihydro-lH-benzimidazole-5-carboxylate (4.0 g, 21 mmol) was mixed with 20 ml of phosphorus oxychloride. The reaction mixture was stirred at 90 C for 1 h and poured into an ice/water slurry. The aqueous mixture was extracted with ethyl acetate. The organic phase was dried over MgS04, filtered and concentrated. The residue was recrystallized first from acetonitrile/water and a second time from toluene/ethyl acetate. A first crop of precipitate was discarded from the toluene/ethyl acetate solution. The title product precipitated after concentrating the mother liquid slightly. Yield: 1.6 g (36%). White solid. MS (ESI+) m/z 211 [M+H]+. HPLC purity: 97%. 1H NMR (600 MHz, DMSO-d6) delta ppm 13.66 (br. s., 1 H) 8.10 (br. s., 1 H) 7.86 (d, J=7.9 Hz, 1 H) 7.61 (br. s., 1 H) 3.87 (s, 3 H). |
- 2
-
 [ 106429-57-6 ]
[ 106429-57-6 ]

-
 [ 683242-75-3 ]
[ 683242-75-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 95% |
|
2-oxo- 2,3-dihydro-1 /-/-benzoimidazole-5-carboxylic acid methyl ester (3.00 g, 15.6 mmol) and phosphorus oxychloride (30 ml_) were combined and heated to 100 0C for 48 h. The mixture was cooled to 23 0C and concentrated under reduced pressure. The residue was cooled to 0 0C, and cold, saturated aqueous NaHCO3 (60 ml_) was added cautiously. After stirring at 23 0C for 15 min, the mixture was sonicated and the resulting residue was filtered to yield the titled compound (3.13 g, 95%), which was used in the next step without further purification. MS (ESI/CI): mass calcd. for C9H7CIN2O2, 210.02; m/z found, 211.0 [M+H]+. |
| 73% |
With trichlorophosphate; at 100℃; for 16h;Inert atmosphere; |
To a solution of methyl 3,4-diaminobenzoate (2 g, 12.0 mmol) in DMF(10 mL) was added l-[(lH-imidazol-l-yl) carbonyl]-lH-imidazole (1.95 g, 12.0 mmol) at room temperature. The mixture was stirred at room temperature under nitrogen atmosphere for 3 h at which time the resulting suspension was filtered. The filter cake was washed with EtOH (20 mL) and dried under vacuum to afford methyl 2-oxo-2,3-dihydro-lH-l,3-benzodiazole-5- carboxylate (1.5 g, 7.8 mmol, yield 65%) as a brown solid: LCMS (ESI) calc'd C9H8N2O3 for [M+H]+: 193, found 193; 1H MR (300 MHz, DMSO-d) delta ppm 11.06 (s, 1H), 10.89 (s, 1H), 7.63 (d, J= 8.2, 1H), 7.47 (s, 1H), 7.02 (d, J= 8.2 Hz, 1H), 3.82 (s, 3H). |
| 72% |
With trichlorophosphate; for 7h;Inert atmosphere; Reflux; |
A suspension of benzimidazolone 2 (25.0 g, 0.130 mol, 1 eq) in POCl3 (150 mL) was heated to reflux for 7 h. The excess of POCl3 was evaporated and the residue was neutralized with saturated NaHCO3 solution. The precipitate was collected by filtration and dried in vacuo. The aqueous phase was extracted with DCM, dried over MgSO4 and the solvent was removed under reduced pressure. The light brown solids obtained this way were of high purity and could be used in the next step without further purification (19.7 g, 93.6 mmol, 72 %). |
Reference:
[1]Patent: WO2009/134750,2009,A1 .Location in patent: Page/Page column 139
[2]Patent: WO2019/55540,2019,A1 .Location in patent: Paragraph 0200
[3]Molecules,2019,vol. 24
[4]Bioorganic and Medicinal Chemistry Letters,2008,vol. 18,p. 5010 - 5014
[5]Cell Chemical Biology,2018,vol. 25,p. 677 - 12,690
- 3
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
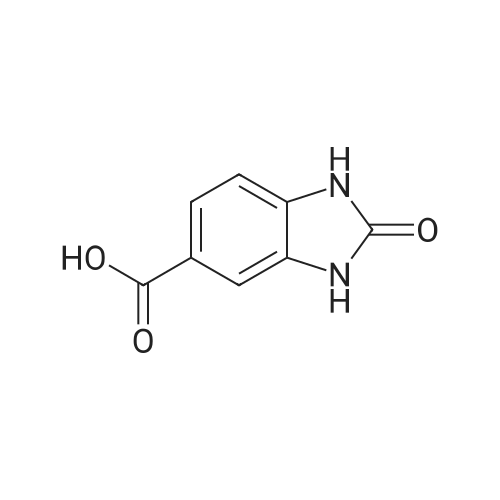 [ 23814-14-4 ]
[ 23814-14-4 ]

-
 [ 683242-75-3 ]
[ 683242-75-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
(1) A mixture of 228 mg of <strong>[23814-14-4]2-oxo-2,3-dihydro-1H-benzimidazole-5-carboxylic acid</strong>, 2.00 mL of phosphorus oxychloride, and one drop of concentrated hydrochloric acid was stirred at 100C overnight. The solvent was removed by distillation under reduced pressure. To the residue was added 4.0 mL of methanol at room temperature, followed by stirring for 1 hour. It was diluted with water and ethyl acetate, and added with potassium carbonate until it had pH 8. The insolubles were separated by filtration, and then extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 313 mg of methyl 2-chloro-1H-benzimidazole-6-carboxylate as a pale brown solid. ESI+: 211 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






