Alternatived Products of [ 6971-11-5 ]
Product Details of [ 6971-11-5 ]
| CAS No. : | 6971-11-5 |
MDL No. : | MFCD00454998 |
| Formula : |
C12H13NO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
203.24
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 6971-11-5 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 6971-11-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 6971-11-5 ]
- 1
-
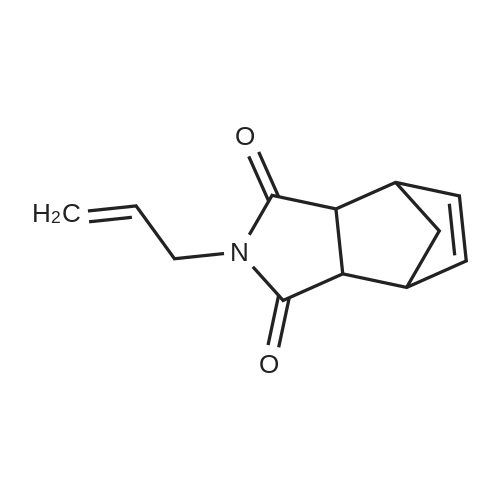 [ 6971-11-5 ]
[ 6971-11-5 ]

-
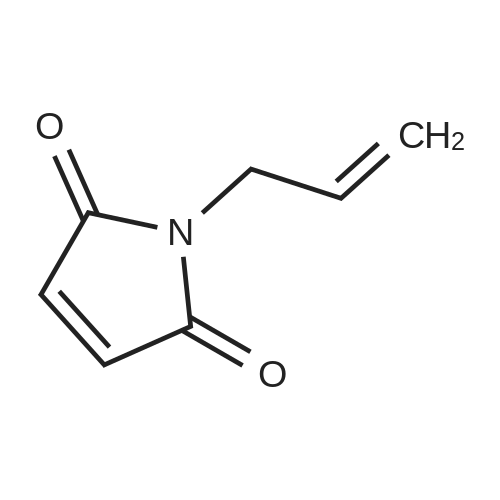 [ 2973-17-3 ]
[ 2973-17-3 ]
- 2
-
 [ 107-11-9 ]
[ 107-11-9 ]

-
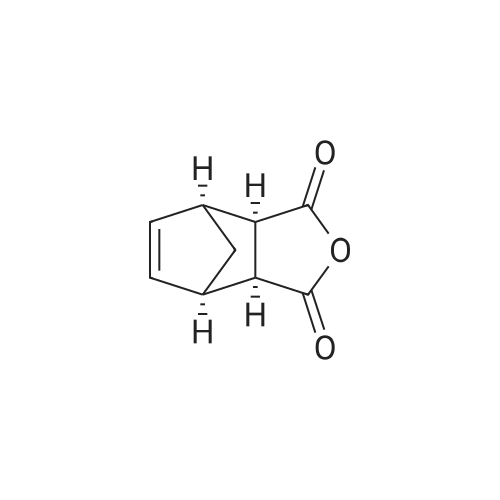 [ 129-64-6 ]
[ 129-64-6 ]

-
 [ 6971-11-5 ]
[ 6971-11-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
In acetic acid for 16h; Inert atmosphere; Reflux; |
|
| 89% |
With acetic acid at 20 - 110℃; for 6h; Reflux; Inert atmosphere; |
2.1 Embodiment 2.
Under nitrogen atmosphere, in 250 ml of adding three-necked bottle 10g (0.102mol) norborneol alkene Shan Gan and 150 ml glacial acetic acid, to be completely dissolved after adding dropwise under stirring 5.82g (0.102mol) ene propyl amine, the reaction at room temperature 1h, then heating to 110 °C reaction refluxing 5h. Stop reaction, to be cooled to the room temperature, is poured into 300 ml water, immediately with a white precipitate out, filtering, the cake is washed with water three times, to obtain white powder product after drying (B1) 12.45g, the yield is 89% |
|
With benzene |
|
Reference:
[1]Gomez-Sanjuan, Asier; Sotomayor, Nuria; Lete, Esther
[European Journal of Organic Chemistry, 2013, # 29, p. 6722 - 6732]
[2]Current Patent Assignee: NANCHANG UNIVERSITY - CN105669738, 2016, A
Location in patent: Paragraph 0026; 0027
[3]Current Patent Assignee: DUPONT DE NEMOURS INC - US2462835, 1943, A
Current Patent Assignee: COMPAGNIE GENERALE DES ETABLISSEMENTS MICHELIN (MICHELIN) - US2524145, 1948, A
- 3
-
 [ 6971-11-5 ]
[ 6971-11-5 ]

-
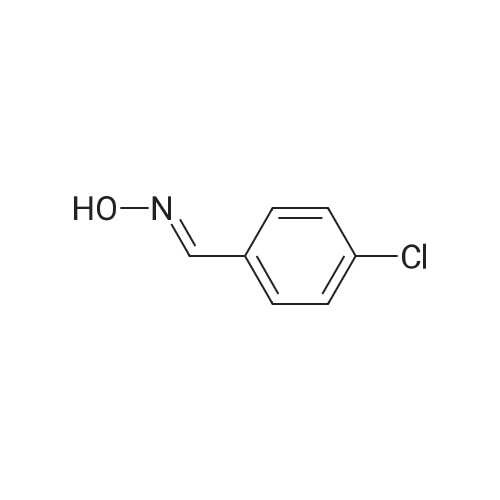 [ 3848-36-0 ]
[ 3848-36-0 ]

-
(3aSR,4SR,4aRS,7aSR,8SR)-6-allyl-3-(4-chlorophenyl)-4,4a,8,8a-tetrahydro-3aH-4,8-methanoisoxazolo[4,5-f]isoindole-5,7(6H,7aH)-dione
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 62% |
With sodium hypochlorite In dichloromethane Cooling with ice; Inert atmosphere; diastereoselective reaction; |
4.1.19. General Procedure for the synthesis of 6a-i
General procedure: A solution of 0.5 mmol of the corresponding oxime, and 0.5 mmol of corresponding dipolarophile in dichloromethane was cooled in an ice bath and 1.3 mmol of sodium hypochlorite as 3.49 mL of 0.35 N solution was added dropwise under stirring within 30 min. The reaction mixture was allowed to stand overnight. The organic layer was separated, the aqueous layer was extracted with dichloromethane, combined organic fractions were dried over Na2SO4. The solvent was removed under reduced pressure and purified by column chromatography on silica gel 40/60, using gradient elution with hexane/ethyl acetate or by crystallization (EtOH) to give the product. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







