Alternatived Products of [ 721-47-1 ]
Product Details of [ 721-47-1 ]
| CAS No. : | 721-47-1 |
MDL No. : | MFCD00034792 |
| Formula : |
C13H12N2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
212.25
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 721-47-1 ]
| Signal Word: | |
Class: | N/A |
| Precautionary Statements: | |
UN#: | N/A |
| Hazard Statements: | |
Packing Group: | N/A |
Application In Synthesis of [ 721-47-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 721-47-1 ]
- 1
-
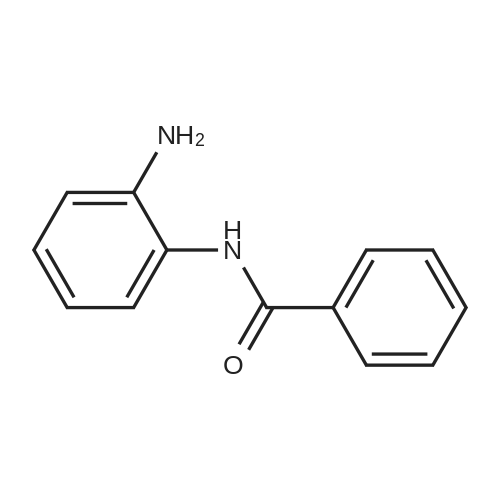 [ 721-47-1 ]
[ 721-47-1 ]

-
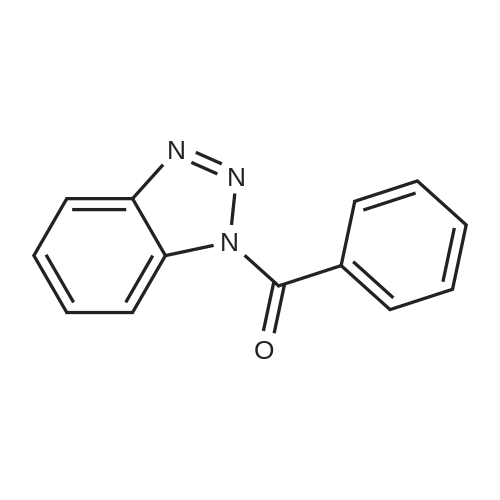 [ 4231-62-3 ]
[ 4231-62-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 95% |
With tert.-butylnitrite In acetonitrile at 27 - 29℃; for 0.25h; |
|
| 75% |
With toluene-4-sulfonic acid In acetonitrile at 0 - 20℃; for 1.5h; Inert atmosphere; |
|
|
With sulfuric acid; sodium nitrite at 0℃; |
|
|
With tert.-butylnitrite In dichloromethane at 25 - 27℃; for 1h; |
|

Reference:
[1]Azeez, Sadaf; Chaudhary, Priyanka; Sureshbabu, Popuri; Sabiah, Shahulhameed; Kandasamy, Jeyakumar
[Organic and Biomolecular Chemistry, 2018, vol. 16, # 37, p. 6902 - 6907]
[2]Faggyas, Réka J.; Sloan, Nikki L.; Buijs, Ned; Sutherland, Andrew
[European Journal of Organic Chemistry, 2019, vol. 2019, # 31-32, p. 5344 - 5353]
[3]Charrier; Beretta
[Gazzetta Chimica Italiana, 1921, vol. 51 II, p. 268]
[4]Sureshbabu, Popuri; Azeez, Sadaf; Chaudhary, Priyanka; Kandasamy, Jeyakumar
[Organic and Biomolecular Chemistry, 2019, vol. 17, # 4, p. 845 - 850]
- 2
-
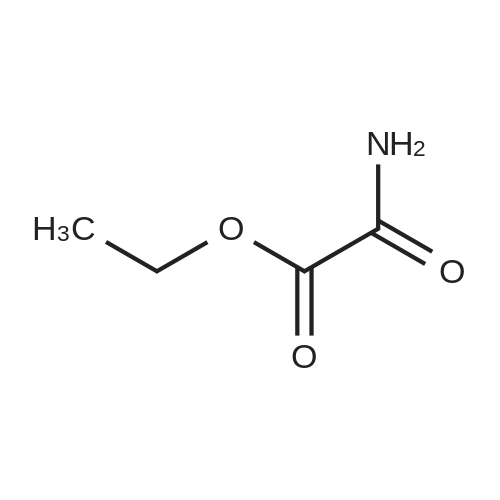 [ 617-36-7 ]
[ 617-36-7 ]

-
 [ 721-47-1 ]
[ 721-47-1 ]

-
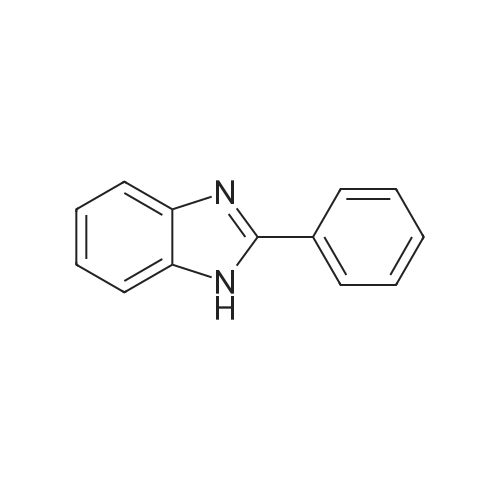 [ 716-79-0 ]
[ 716-79-0 ]
- 3
-
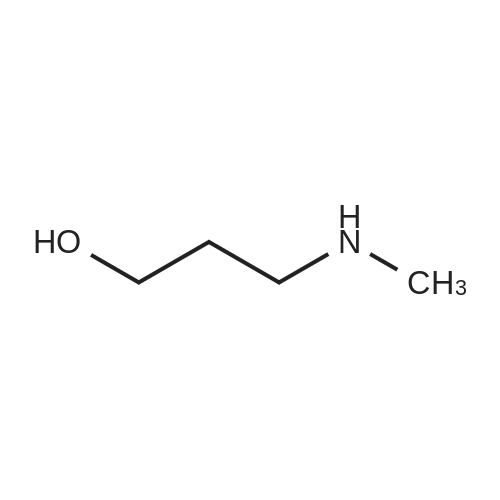 [ 42055-15-2 ]
[ 42055-15-2 ]

-
 [ 39827-11-7 ]
[ 39827-11-7 ]

-
 [ 721-47-1 ]
[ 721-47-1 ]

-
benzo[<i>b</i>]thiophene-2-carboxylic acid (2-benzoylamino-phenyl)-(3-methylamino-propyl)-amide
[ No CAS ]
- 4
-
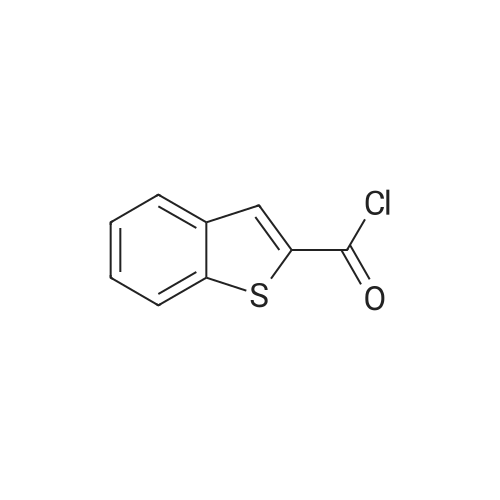
 [ 879-65-2 ]
[ 879-65-2 ]

-
 [ 721-47-1 ]
[ 721-47-1 ]

-
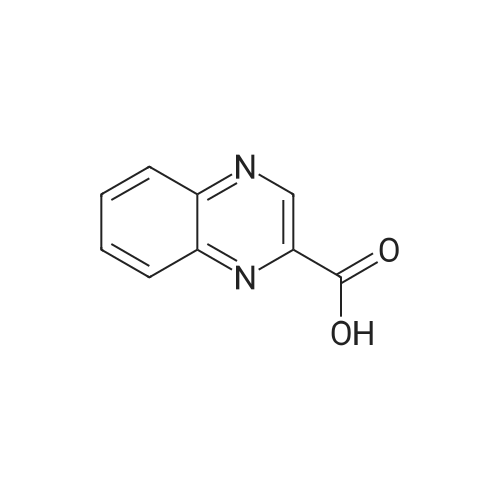
 [ 1257862-88-6 ]
[ 1257862-88-6 ]
- 5
-
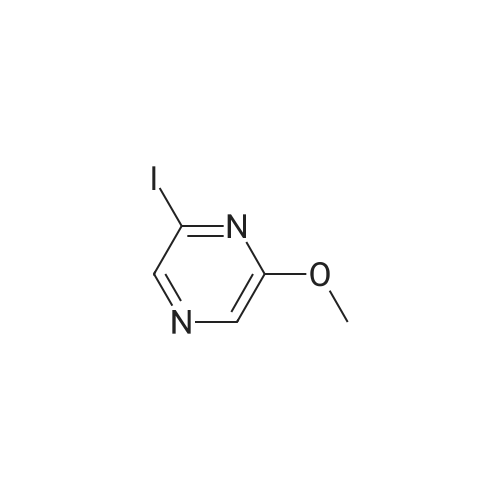 [ 58139-03-0 ]
[ 58139-03-0 ]

-
 [ 721-47-1 ]
[ 721-47-1 ]

-
N-(2-aminophenyl)-2-(6-methoxypyrazin-2-yl)benzamide
[ No CAS ]
- 6
-
 [ 4231-62-3 ]
[ 4231-62-3 ]

-
 [ 95-54-5 ]
[ 95-54-5 ]

-
 [ 721-47-1 ]
[ 721-47-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 82% |
In butan-1-ol at 25℃; for 3h; |
General procedure for monoacylationof symmetrical aromatic diamines in n-butanol
General procedure: In a round bottom flask, p-phenylenediamine (2a) or o-phenylenediamine(2b) (7.5 mmol), were dissolved in 5 cm3 ofn-butanol at 25 °C. The corresponding N-acylbenzotriazole1a-1g (5 mmol) was then added to the solution and the mixturewas stirred at room temperature for 3 h. Upon completionof the reaction (monitored by TLC ethylacetate/hexane1:1) n-butanol was evaporated. The semisolid was dissolvedin 20 cm3 ethyl acetate and the organic layer was washedwith saturated Na2CO3(3 × 5 cm3), water (2 × 5 cm3), and5 cm3 brine. After evaporation of ethyl acetate under reducedpressure, the solid separated was dried under vacuum to givethe desired monoacylated products (Table 1). |
- 7
-
 [ 766-96-1 ]
[ 766-96-1 ]

-
 [ 721-47-1 ]
[ 721-47-1 ]

-
(2-(4-bromobenzyl)-1H-benzo[d]imidazol-1-yl)(phenyl)methanone
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 88.7% |
With copper(l) iodide; 4-toluenesulfonyl azide; tri-tert-butylamine In acetonitrile at 60℃; for 12h; |
4; 8; 12; 16; 20; 24; 28 Example 4
General procedure: To acetonitrile, add the above formula (II), (III) and (IV) compounds, cuprous iodide (CuI),Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), then the temperature was raised to 60°C, and the reaction was stirred at this temperature in the air for 12 hours.Among them, the molar ratio of the compound of formula (II) to cuprous iodide (CuI) is 1:0.4; the compound of formula (II) and tris[(1-benzyl-1H-1,2,3-triazole-4- The molar ratio of methyl)amine (TBTA) is 1:2;The molar ratio of the compound of formula (II) to the compound of (III) and (IV) is 1:3:3; and the compound of formula (II) in millimoles (mmol) and acetonitrile in milliliters (ml) The ratio is 1:15.After the reaction is complete, the reaction system is naturally cooled to room temperature,The solvent was distilled off under reduced pressure to obtain the crude product. The crude product was subjected to 200-300 mesh silica gel column chromatography, using a mixture of ethyl acetate and petroleum ether as the eluent, and the volume ratio of ethyl acetate to petroleum ether was 1:15. Thus, the target compound of formula (I) (C21H15BrN2O) was obtained as a yellow liquid, with a yield of 80% and a purity of 98.7% (HPLC). |
| 80% |
With copper(l) iodide; 4-toluenesulfonyl azide; triethylamine In acetonitrile at 80℃; for 3.5h; |
|
Reference:
[1]Current Patent Assignee: GUANGDONG LABORATORY OF SOUTHERN OCEAN SCIENCE AND ENGINEERING (ZHANJIANG) - CN113214162, 2021, A
Location in patent: Paragraph 0056-0063; 0064-0081
[2]Yang, Weiguang; Zhao, Yu; Zhou, Zitong; Li, Li; Cui, Liao; Luo, Hui
[RSC Advances, 2021, vol. 11, # 15, p. 8701 - 8707]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping














