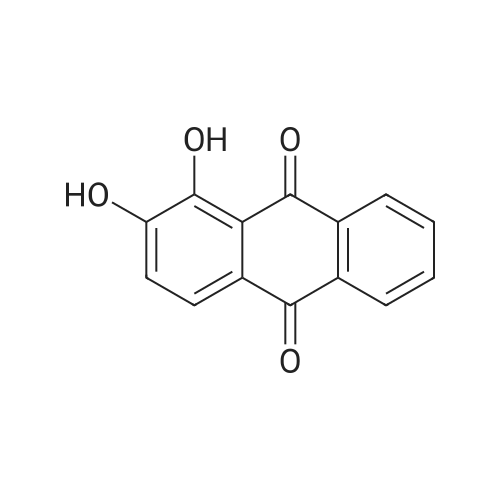
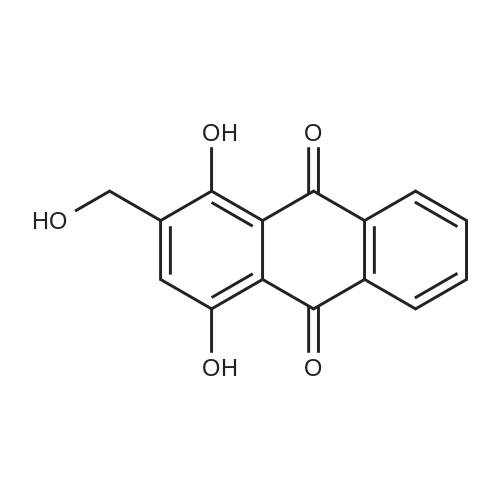
|
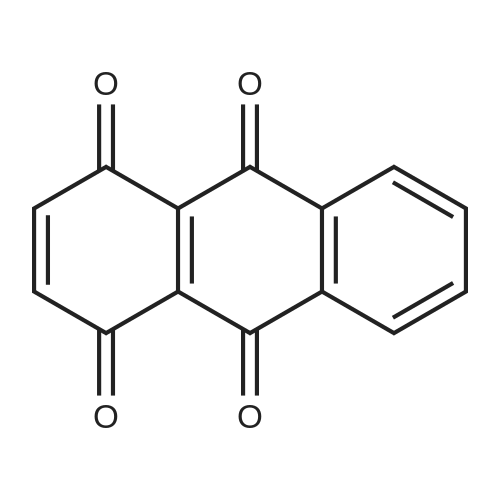
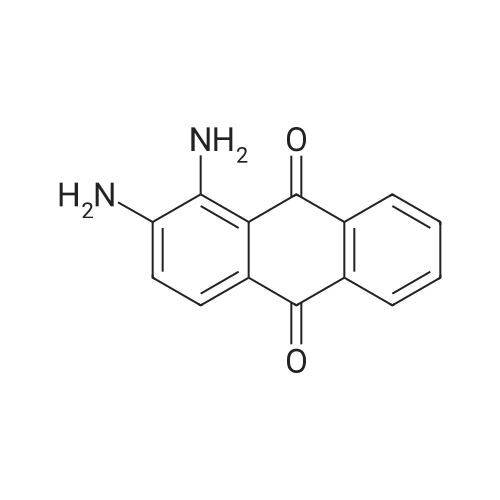
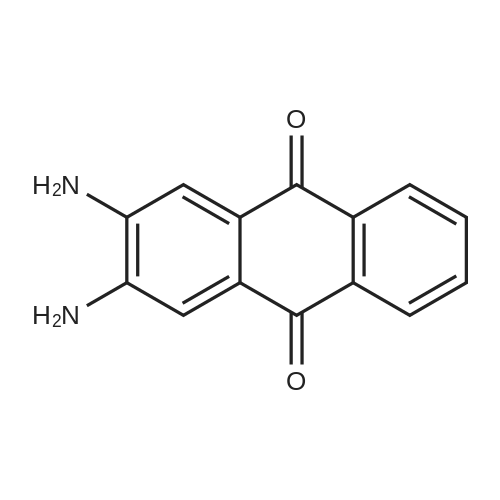
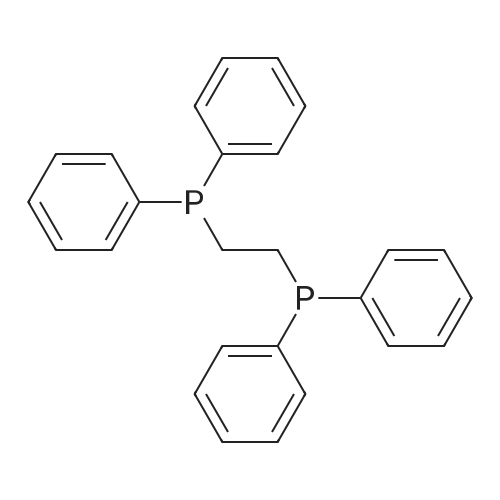
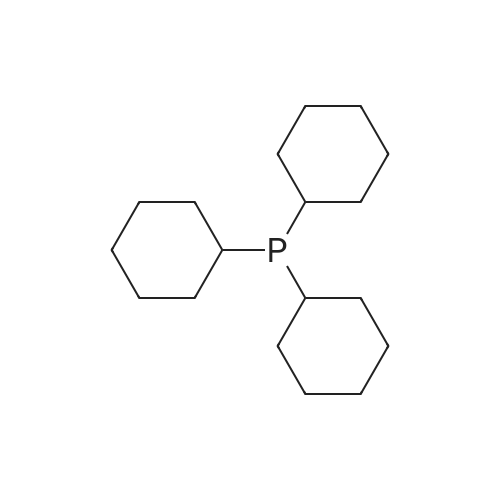
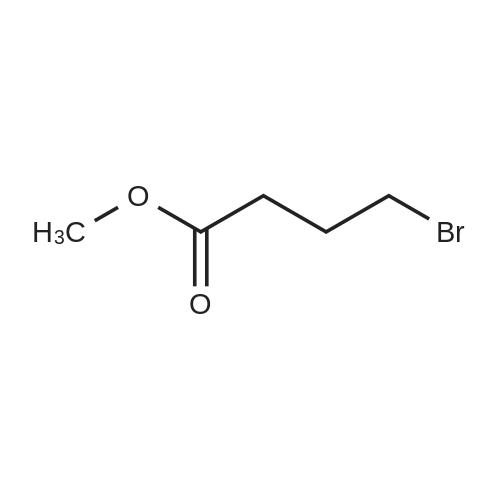
In 1,3,5-trimethyl-benzene;Heating / reflux; |
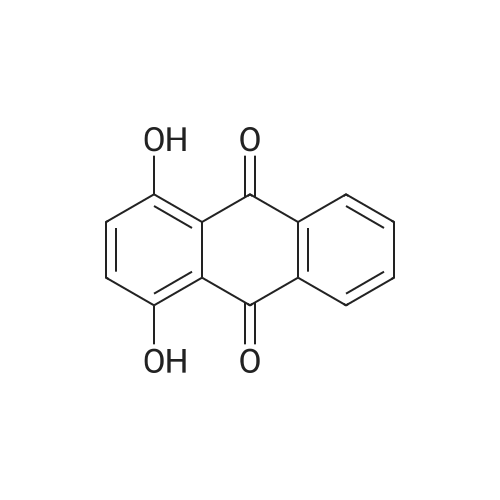
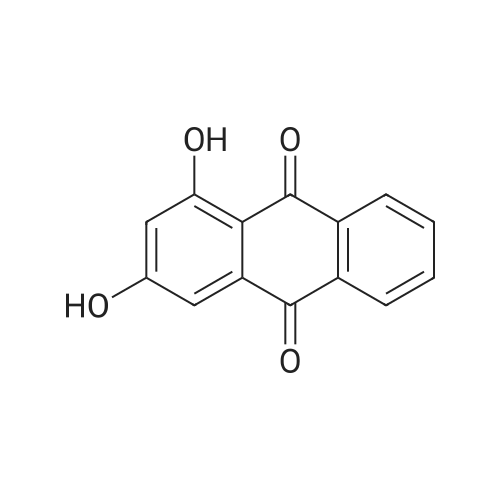
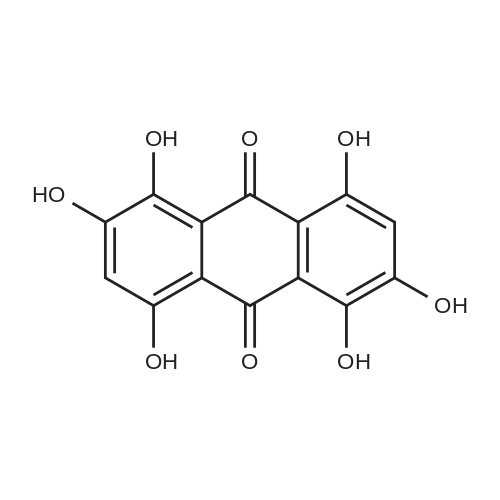
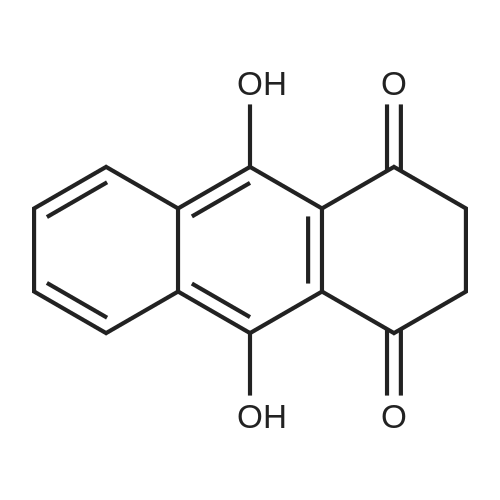
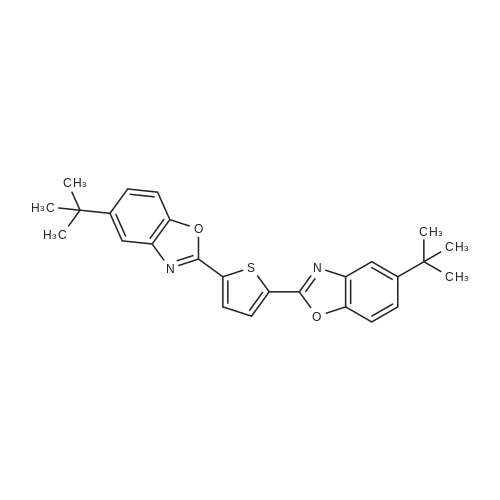
Herein we report on a new orthogonal-bonding approach for assembling functional molecules. This approach, which is an offshoot of the directional-bonding approach, involves the simultaneous introduction of a bis(chelating) dianion to coordinate to two equatorial sites of two fac-(CO)3Re cores and a ditopic nitrogen-donor ligand to the remaining orthogonal axial site, leading to the generation of a new, hitherto unexplored class of metallacycles (FIG. 3).2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene (tpbb) and 1,4-dihydroxy-9,10-anthraquinone (H2-dhaq) or 1,2,4-trishydroxy-9,10-anthraquinone (H2-thaq) were chosen for use as basic building units. The presence of two nitrogen donors should permit the tpbb ligand to act as a neutral bifunctional molecular clip. We rationalized that the use of the bischelating ligands H2-dhaq and H2-thaq would result in a macrocycle host with a large internal cavity. Although both ligands are important in dye chemistry, electroluminescent devices, biology, and pharmaceutical chemistry, their use as building units in the construction of supramolecular assemblies has not been previously investigated. The combination of the above-mentioned new building blocks and the novel orthogonal-bonding approach permits the preparation of unique gondola-shaped structures with crown-ether-like recognition sites, highly fluorescent properties, and selective binding capabilities. This approach is so effective that the products can be prepared in near quantitative yield.The assembly of compounds 3 and 4 was achieved by reacting equimolar amounts of Re2(CO)10, tpbb, and H2-dhaq or H2-thaq in refluxing mesitylene. The resulting dark-green products were air and moisture stable and are slightly soluble in polar solvents. The IR spectrum of 3 in acetone exhibited strong bands at 2014 and 1900 cm-1, characteristic of fac-Re(CO)3. The 1H NMR spectrum of 3 showed well-resolved signals for each of the protons. Compared to the free ligands, the signals corresponding to the tpbb protons remained nearly unchanged, while those of the dhaq proton of H2 was shifted upfield by 0.16 ppm after complexation with the Re(I) centers. A similar pattern was observed for 4 with an additional singlet at 10.99 ppm corresponding to the uncoordinated hydroxyl hydrogen (C2-OH) atoms. The ESI-MS spectrum of 3 showed a molecular ion peak at m/z 2418.1.A single-crystal X-ray diffraction analysis shows that compound 3 adopts an unusual gondola-shaped structure (FIG. 4). The structure can be regarded as a special type of grid. The two tpbb ligands serve as molecular clips, utilizing the benzoxazoline N atoms to bridge two doubly bridged dirhenium units. The bishydroxy anthraquinone (dhaq) acts as a doubly bridging unit using the adjacent phenolate and quinone oxygens. Interestingly, the hydrophobic internal cavity of the metallacycle is sufficiently large (size: 5.6 ×7.0 ×17.8 ) to accommodate four MeOH molecules. Compound 4 is isostructural with respect to 3 but contains two additional uncoordinated hydroxyl groups. It is noteworthy that compounds 3 and 4 possess multiple-recognition sites. The arrangement of heteroatoms may be considered as the structural framework of 1,10-dithio-(18crown-6) (see Supporting Information).Compound 3 in CH2Cl2 displayed intense absorption bands in the 230-395 nm region, which are assigned to ?-?* transitions of the dhaq and tpbb (357, 378, 397 nm) ligands, and a weak shoulder at 420 nm, assigned to the MLCT transition (Re?tpbb). In addition, weak absorption bands appeared at 585 and 632 nm, attributed to an intraligand transition of the dhaq unit. Compounds 3 and 4 show a high luminescence at room temperature with a quantum yield of 0.179 for 3 and 0.397 for 4 relative to Ru(bpy)32+. Upon excitation at lambdamax=378 nm, compound 3 emits a set of structured bands centered at 438 nm with a lifetime of 1.4 ns. These emission bands are similar to that of the tpbb ligand. The small Stokes shift and very short lifetime of 3 indicate that the emission originates from the singlet ?-?* excited state. In the solid state, compound 3 exhibits two emission maxima at 448 and 518 nm when excited at 335 nm. The emission band at 448 nm is due to the decay of the ?-?* excited state of tpbb, while the band at 518 nm may be attributed to the decay of the d-?*Re tpbb excited state. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping