|
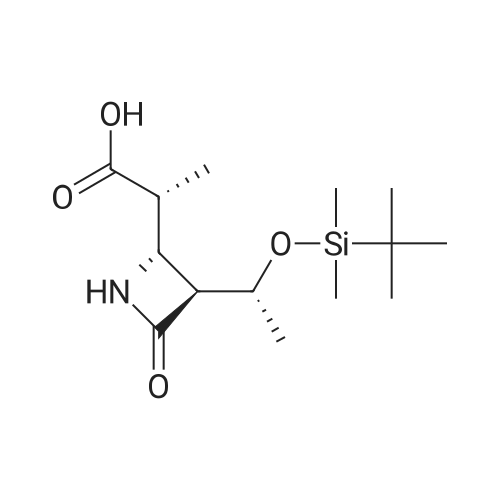
Stage #1: (2R)-2-[(2S,3S)-3-{(1R)-1-(t-butyldimethylsilyloxy)ethyl}-4-oxoazetidin-2-yl]propionic acid With 1,1'-carbonyldiimidazole In acetonitrile at 20℃; for 0.5h;
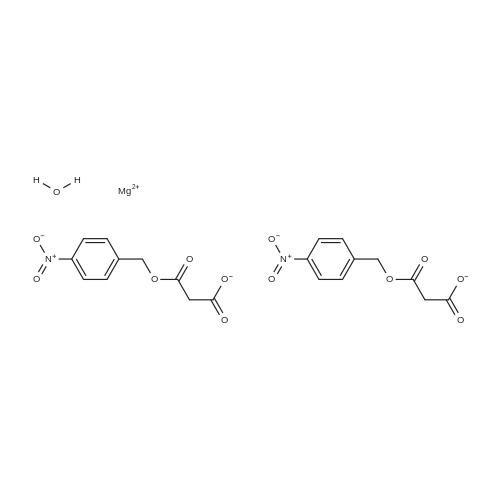
Stage #2: magnesium mono-p-nitrobenzyl malonate In acetonitrile at 25 - 35℃; for 18h; |
2
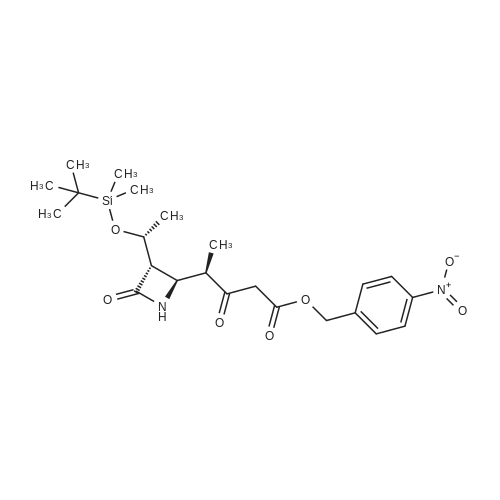
Example 2; Synthesis of (3S,4R)-3-[(lR)-l-hydroxyethyl] -4-[(lR)-l-methyl-3-diazo-4- [(4- nitrobenzyl)oxy]-2,4-dioxobutyl]azetidin-2-one (IX)Compound (VI) (25g, 86mmol) was added into anhydrous acetonitrile (300ml). After the addition of carbonyldiimidazole (17.5g, llOmmol) under stirring, the resultant mixture was stirred continuously at the room temperature for 30 min. Subsequently, anhydrous magnesium mono-p-nitrobenzyl malonate (55.5g, llOmmol) was added, and then the resultant reaction mixture was stirred at 25 to 35 °C for 18 hr. After the reaction was completed, ethyl acetate (450ml) and IN hydrochloric acid (450ml) were added under stirring to adjust the pH of the aqueous phase within the range of 2 to 3. The organic phase was washed thoroughly with brine, 5% solution of potassium carbonate in water, and brine successively to get a solution of (3S,4R)-3-[(lR)-l-t-butyldimethylsilyloxyethyl]-4-[(lR)-l-methyl-4-[(4-nitrobenzyl)oxy]-2,4-d ioxobutyl]azetidin-2-one (VII) in ethyl acetate, which may be used for the next step without isolation. |
|
Stage #1: (2R)-2-[(2S,3S)-3-{(1R)-1-(t-butyldimethylsilyloxy)ethyl}-4-oxoazetidin-2-yl]propionic acid With 1,1'-carbonyldiimidazole In acetonitrile at 20℃; for 0.5h;
Stage #2: magnesium mono-p-nitrobenzyl malonate In acetonitrile at 25 - 35℃; for 18h; |
4
[Example 4]; Synthesis of (3S,4R)-3-[(lR)-l-hydroxyethyl] -4-[(lR)-l-methyl-3-diazo-4- [(4- nitrobenzyl)oxy] -2,4-dioxobutyl] azetidin-2-one (IX) (3S,4S)-4-[(lR)-l-methyl-l-carboxyethyl]-3-[(lR)-l-(t-butyldimethylsilyloxy) ethyl] -2-azetidinone (VI) (25g, 86mmol) was added to anhydrous acetonitrile (300 ml), to which was added carbonyldiimidazole (17.5g, llOmmol) with stirring. After the mixture was stirred at room temperature for 30 min, anhydrous magnesium mono-p-nitrobenzyl malonate (55.5 g, 110 mmol) was added. The reaction mixture was stirred at 25-35 °C for 18 h. After completion of the reaction, to the reaction mixture was added ethyl acetate (450 ml) and hydrochloric acid (450 ml, IN) to acidify the aqueous phase at pH 2-3. The organic phase was washed successively with brine, aqueous potassium carbonate solution (5%), and brine. The ethyl acetate solution of (3S,4R)-3-[(lR)-l-t-butyldimethylsilyloxyethyl]-4-[(lR)-l-methyl-4-[(4- nitrobenzyl)oxy]-2,4-dioxobutyl]azetidin-2-one(VII) is obtained. The product could be used in the next step without separation.To the above ethyl acetate solution of(3S,4R)-3-[(lR)-l-t-butyldimethylsilyloxyethLyl]-4-[(lR)-l-methyl-4-[(4-nitrobenzyl)oxy]-2,4-di oxobutyl]azetidin-2-one( (VII) was added methanol (100 ml), then added hydrochloric acid (100 ml, 6N) at 20-250C. The reaction mixture was stirred at the same temperature for 2 h. After completion of the reaction, saturated brine (500 ml) was added. The organic phase was further washed with sodium phosphate dibasic (10%, 2x500ml), then washed with saturated brine, dried with anhydrous magnesium sulfate, and condensed under vacuum. The concentrate was mainly (3S,4R)-3-[(lR)-l-hydroxyethyl]-4-[(lR)-l-methyl-4-[(4-nitrobenzyl)oxy]-2,4-dioxobutyl]azetid in-2-one (VIII). The product could be used in the next step without separation. The above concentrate was added to acetonitrile (140 ml) and stirred to dissolve.Dodecylbenzene sulfonyl azide (30.3 g, 86 mmol) and triethylamine (9.6g, 95mmol) were then added and the reaction mixture was stirred for 2 hours. The organic phase was washed with hydrochloric acid (220 ml, 0.5 N), and then washed thoroughly with water and brine, dried with anhydrous magnesium sulfate, and concentrated under vacuum to give oil. Purification by column chromatography (elution: ethyl acetate : petroleum ether = 2 : l(v:v)) on silica gel (250 ml) gave (3S,4R)-3-[(lR)-l-hydroxyethyl] -4-[(lR)-l-methyl-3-diazo-4- [(4- nitrobenzyl)oxy]-2,4-dioxobutyl]azetidin-2-one (IX) (25g, 77.2% yield) as pale yellow crystal. The physical data of compound (I): [ α ]D21=-50.4° (c=2.5, CH2Cl2) 2140,1750, lq720, 1650.NMR S (CDCl3): 1.22(3H, d, J=6.0 Hz), 1.32(3H, d, J=6.0 Hz),2.38(lH, d, J=3.2 Hz), 2.92QH, dd, J=2.4, 7.6 Hz), 3.77 (IH, m), 3.86 (IH, m), 4.15(1H, m), 5.38(2H, s).5.90(lH,s), 7.57and 8.30(2H, m). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping