Alternatived Products of [ 847795-89-5 ]
Product Details of [ 847795-89-5 ]
| CAS No. : | 847795-89-5 |
MDL No. : | N/A |
| Formula : |
C15H25NO3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
267.36
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 847795-89-5 ]
| Signal Word: | |
Class: | N/A |
| Precautionary Statements: | |
UN#: | N/A |
| Hazard Statements: | |
Packing Group: | N/A |
Application In Synthesis of [ 847795-89-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 847795-89-5 ]
- 1
-
 [ 24424-99-5 ]
[ 24424-99-5 ]

-
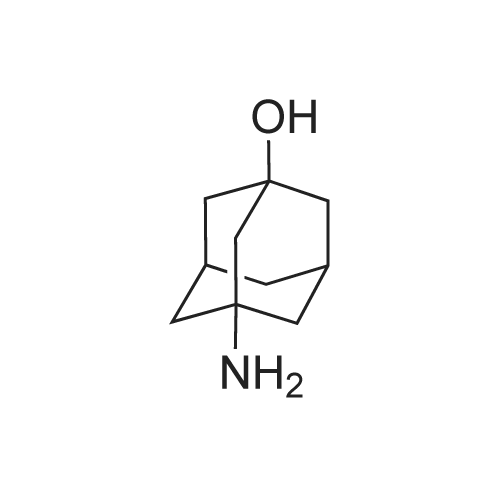 [ 702-82-9 ]
[ 702-82-9 ]

-
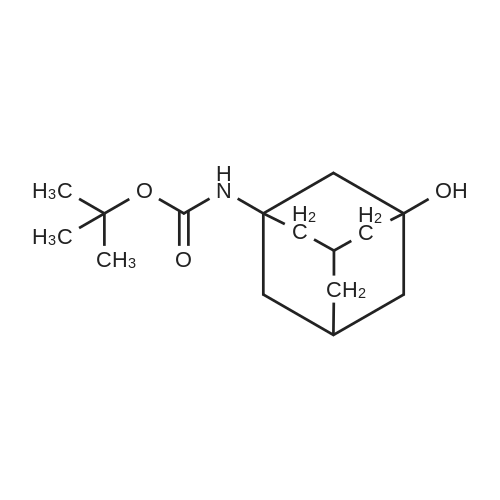 [ 847795-89-5 ]
[ 847795-89-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 65% |
With sodium hydroxide; In 1,4-dioxane; water; at 20℃; for 16h; |
a. ) TERT-BRTYL [3- (P R IMIDIN-2-VLAMINO)-1-ADAMANTVL] CARBAMATE; In general formula (V) the meaning of R and B are as defined above, Y represents tert- butoxycarbonyl group.; (i) TERT-BUTVL (3-HYDROXV-1-ADAMANTYL) CARBAMATE; 2.51 g (15 mmole) of 3-hydroxy-1-aminoadamantane (Pharm. Chem. J. (Engl. Trans.) 1990, 24, 35) are dissolved in the mixture of 15 ml OF DIOXANE, 15 ml of water and 15 ml of 1N sodium hydroxide solution, then under cooling and stirring 4.91 g (22.5 mmole) OF DI- TERT-BUTYL dicarbonate are added. The mixture is stirred at room temperature for 16 hours, the solution is evaporated, and the residue is dissolved in the mixture of 50 ml of ethyl acetate and 50 ml of water. Following extraction and separation of the phases, the organic phase is dried over sodium sulphate. After evaporation the white crystalline residue is treated with n-hexane, to obtain 2.61 g (65%) of product. M. p.: 131-132C. 1H-NMR. : (DMSO-d6) : 1.36 (s, 9H), 1.41 (s, 2H), 1.48 (d, 4H), 1.70 (d, 6H), 2.10 (bs, 2H), 4.43 (s, 1H), 6.41 (s, 1H). |
|
With triethylamine; In tetrahydrofuran; at 20℃;Cooling with ice; |
8-2 (167 mg, 1 mmol) was dissolved in 2 ml of THF, 164 mg of di-tert-butyl dicarbonate was added under ice-cooling, and then 14 ml of triethylamine was gradually added dropwise to room temperature and allowed to react overnight. The THF was evaporated to dryness and extracted with 10 ml of ethyl acetate. The organic layer was washed with brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give crude 8-3 (230 mg, 86%) as a white solid. The intermediate8-3 (1 eq) was dissolved in 3 ml of dichloroethane,DMAP (0.1 eq) was added,Dropping butanoyl chloride under stirring,After reaction for 2 h,The solvent was evaporated to dryness, and water was added thereto. The mixture was extracted with methylene chloride. The methylene chloride layer was washed with saturated brine and dried. The solvent was distilled off under reduced pressure, and the residue was purified by column chromatography to give Intermediate 8-4. The resulting intermediate 8-4 was dissolved directly in methylene chloride and trifluoroacetic acid was added with stirring. After 1 h, the reaction solution was dried to give intermediate 8-1. |
- 2
-
 [ 847795-89-5 ]
[ 847795-89-5 ]

-
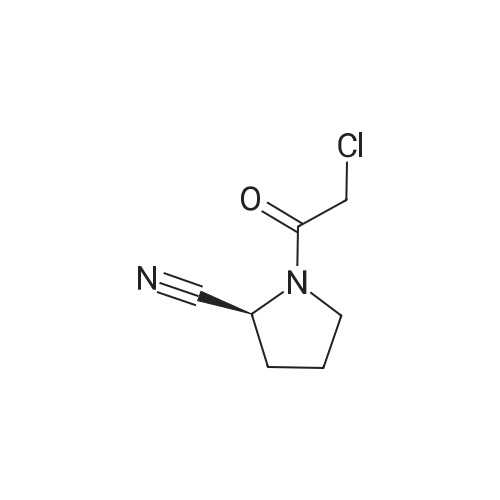 [ 207557-35-5 ]
[ 207557-35-5 ]

-
1-[2-(3-(Boc-amino)adamantane-1-oxy)acetyl]pyrrolidine-2(S)-carbonitrile
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 82.5% |
With triethylamine; In dichloromethane; at 50℃; for 3h; |
Take 18.9 g of Compound I (0.11 mol),26.7 g of compound II (0.1 mol),15 g of triethylamine (0.15 mol),500 mL of dichloromethane, placed in a 1000 mL three-necked flask,Stir, heat to 50 C, reaction for 3 h,TLC monitoring, the reaction is completed,Add 100 mL of water and dispense.The methylene chloride phase was taken, dried over anhydrous magnesium sulfate, and the desiccant was filtered off.The organic phase is concentrated to a white solid.That is, compound III was 33.2 g, and the yield was 82.5%. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






