| 96% |
With pyridine; In dichloromethane; at 20℃; for 24h; |
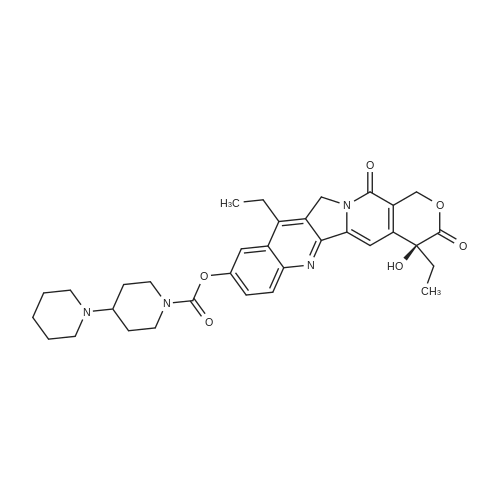
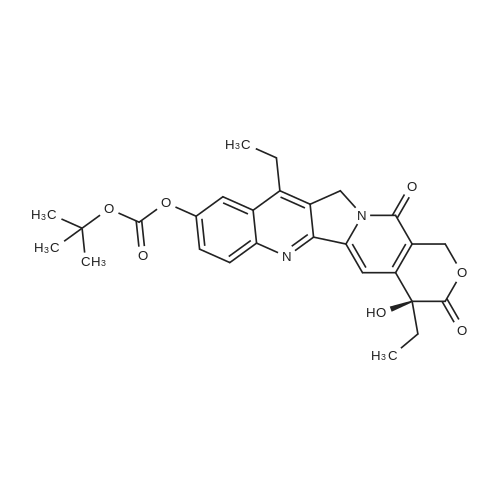
1003861 To a solution of <strong>[86639-52-3]SN-38</strong> (1.64 g, 4.18 mmol) in dichloromethane (15 mL)and pyridine (3.04 mL, 37.6 mmol) was added di-tert-butyl dicarbonate (1.82 g, 8.36mmol). The reaction was stirred at room temperature for 24 h, and all solvent wasremoved under vacuum. The remaining residue was redissolved in dichloromethane(20 mL), and this solution loaded onto an 80 g silica gel column. Eluting with 0% to10% methanol in dichloromethane provided Boc-<strong>[86639-52-3]SN-38</strong> (1.98 g, 4.02 mmol, 96%yield). |
| 95.2% |
With pyridine; In dichloromethane; at 20℃; |
Compound 2 was prepared according to the method, with some modifications [31]. Briefly, di-tert-butyldicarbonate (2.7 g, 12.37mmol) and pyridine (25 ml) were added in a suspension of SN38 (9.17 mmol) in 360 ml CH2Cl2. The suspension was stirred overnight at room temperature. The mixture was washed with 0.5 N HCl (3100 ml) and saturated NaHCO3 (1100 ml). The organic phase was filtered through a Na2SO4 pump, dried, and evaporated at 30C under vacuum. The solids obtained were dried under vacuum at 50C to yield compound 2 (4.3 g, 95.2% yield). |
| 95.2% |
With pyridine; In dichloromethane; at 20℃; |
Compound 8 was prepared according to the method that was described in our previous studies [41]. Briefly, di-tertbutyldicarbonate(2.7 g) and pyridine (25 ml) were added to the suspension of <strong>[86639-52-3]SN-38</strong> (3.6 g) in 360 ml CH2Cl2. The suspension was stirred overnight at room temperature. The mixture was washed with 0.5 N HCl (3 × 100 ml)and saturated NaHCO3 (1 × 100 ml). The organic phase was filtered, dried over Na2SO4, evaporated at 30C under vacuum. The obtained solids were dried under vacuum at 50C to give compound 7 (4.3 g,95.2% yield). (M + H)+ = 493.1989; (M + Na)+ = 515.1796; 1HNMR (400 MHz, CDCl3): delta 1.04 (3H, t, J = 7.2 Hz), 1.41 (t, 3H, J =7.6 Hz), 1.61 (s, 9H), 1.85-1.93 (m, 2H), 3.17(q, 2H, J = 7.6 Hz), 3.81(brs, 1H),5.27 (s, 2H), 5.31 (d, 1H, J = 16.4 Hz), 5.75 (d, 1H, J =16.0 Hz), 7.67 (dd, 1H, J = 2.4, 9.2 Hz), 7.72 (s,1H), 7.91 (d,1H, J =2.4 Hz), 8.28 (d,1H, J=9.2Hz). |
| 90% |
With pyridine; In dichloromethane; at 20℃; for 3h; |
1 g (2.5 mmol) SN38 and 1 g (4.5 mmol) Di-tert-butyl dicarbonate were dissolved in CH2Cl2 and 5 mLpyridinewas used as a catalyst. After stirring for 3 h at room temperature, the reaction mixture was washed by aqueous hydrochloric acid solution and then dried over anhydrous MgSO4 to obtain 1.2 g Boc-SN38 in 90% yield, as characterized by 1H NMR spectroscopy (Fig. S2). |
| 86% |
With pyridine; In dichloromethane; at 20℃; for 12h; |
To a solution of (S)-4,1 1 -diethyl-4, 9-dihydroxy- 1 ,12-dihydro-14H-pyrano[3',4':6,7]indolizino[1 ,2- b]quinoline-3,14(4H)-dione (2.5 g, 6.37 mmol, 1 equiv.) in DCM (15 ml_) was added B C O (2.09 g, 9.56 mmol, 2.20 mL, 1 .5 equiv.) and pyridine (1 .01 g, 12.7 mmol, 1 .03 mL, 2 equiv.) at 20 C. The mixture was stirred at 20 C for 12 hr. The reaction mixture was concentrated under reduced pressure to give (S)-tert- butyl (4,1 1 -diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1 H-pyrano[3',4':6,7]indolizino[1 ,2-b]quinolin- 9-yl) carbonate (2.7 g, 86%) as a white solid. |
| 82% |
With pyridine; In dichloromethane; at 20℃; |
Example 8 Boc-(10)-(<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong>) (Compound 10) To a suspension of <strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (compound 4, 2.45 g, 1 eq.) in 250 mL of anhydrous DCM at room temperature under N2 were added di-tert-butyl dicarbonate (1.764 g, 1.3 eq.) and anhydrous pyridine (15.2 mL, 30 eq.). The suspension was stirred overnight at room temperature. The hazy solution was filtered through celite (10 g) and the filtrate was washed with 0.5 N HCl three times (3*150 mL) and a NaHCO3 saturated solution (1*150 ml). The solution was dried over MgSO4 (1.25 g). The solvent was removed under vacuum at 30 C. The product was dried under vacuum at 40 C. (yield=82%, 2.525 g) 13C NMR (75.4 MHz, CDCl3) d 173.53, 157.38, 151.60, 151.28, 150.02, 149.70, 147.00, 146.50, 145.15, 131.83, 127.19, 127.13, 124.98, 118.53, 113.88, 98.06, 84.26, 72.80, 66.18, 49.33, 31.62, 27.73, 23.17, 13.98, 7.90. |
| 82% |
With pyridine; In dichloromethane; at 20℃;Inert atmosphere; |
EXAMPLE 8. Boc-(10)-(<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong>) (compound 10):; To a suspension of 7-ethyl-lO-hydroxycamptothecin (compound 4, 2.45 g, 1 eq.) in 250 mL of anhydrous DCM at room temperature under N2 were added di-tert-butyl dicarbonate (1.764 g, 1.3 eq.) and anhydrous pyridine (15.2 mL, 30 eq.). The suspension was stirred overnight at room temperature. The hazy solution was filtered through celite (10 g) and the filtrate was washed with 0.5 N HCl three times (3 x 150 mL) and a NaHCO3 saturated solution (1 x 150ml). The solution was dried over MgSO4 (1.25 g). The solvent was removed under vacuum at 30 C. The product was dried under vacuum at 40 C (yield = 82%, 2.525g) 13C NMR (75.4 MHz, CDCI3) 8 173.53, 157.38, 151.60, 151.28, 150.02, 149.70, 147.00, 146.50, 145.15, 131.83, 127.19, 127.13, 124.98, 118.53, 113.88, 98.06, 84.26, 72.80, 66.18, 49.33, 31.62, 27.73, 23.17, 13.98, 7.90. |
| 81% |
With pyridine; In dichloromethane; at 20℃; |
Take Boc2O (361.5 mg, 1.66 mmol), SN38 (500 mg, 1.28 mmol) dissolved in 8 mL DCM.2 mL of pyridine was added and allowed to stand overnight at room temperature, followed by HCl (0.3 M) aqueous solution.The organic layer was dried over anhydrous sodium sulfate and filtered.After collecting the filtrate, the solvent was removed under reduced pressure; the solid was purified by column chromatography (dichloromethane:methanol=100:1).The objective product 4 (510 mg, yield 81%) was obtained. |
| 80% |
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 50℃; for 4h; |
(Boc)2O (145 mg, 0.66 mmol) and SN38 (200 mg, 0.51 mmol) were added to a 100 mL round bottom flask, dissolved in 15 mL of anhydrous dichloromethane and EDC (118.8 mg, 0.77 mmol) was added. After stirring at 50 C for 4 hours, the reaction solvent was removed and washed with saturated sodium bicarbonate and saturated brine, respectively. The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was collected and the solvent was removed under reduced pressure. The residue 7a, Boc-SN38 (180 mg, 80%) was isolated by column chromatography (DCM: MeOH=120:1). |
| 80% |
With pyridine; In dichloromethane; at 20℃; |
2.0g<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (SN38),1.0 g of di-tert-butyl dicarbonate was suspended and suspended in 150 mL of anhydrous dichloromethane.8 mL of anhydrous pyridine was added dropwise, and the reaction was stirred at room temperature overnight. filter,The filtrate was washed three times with 0.5 M hydrochloric acid and once with saturated sodium bicarbonate solution.The organic phase was separated and dried over anhydrous magnesium sulfate. After drying, remove the solvent in vacuo.There was obtained 10-tert-butyl carbonate-7-ethyl-camptothecin (BOC-SN38, 1.22 g, 80%). |
| 48% |
pyridine; In N,N-dimethyl-formamide; at 20℃; for 12h; |
Example 7: Synthesis and In vitro Analysis of CDP-Gly-<strong>[86639-52-3]SN-38</strong><strong>[86639-52-3]SN-38</strong> was derivatized with the amino acid glycine at the 20-OH position as shown in Scheme VIII. Briefly, 20(S)-<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (<strong>[86639-52-3]SN-38</strong>, l.Og, 2.5 mmol) was dissolved in a mixture of 70 mL dimethylformamide (DMF) and 30 mL pyridine. A solution of di-tert-butyl-dicarbonate (0.83 g, 3.8 mmol) in 10 mL DMF was added and the mixture stirred at room temperature overnight (12 hours). The solvent was removed under vacuum to yield a yellow solid and re-crystallized from boiling 2-propanol (75 mL) to yield 20(s)-10-tert-butoxycarbonyloxy-7-ethyl- camptothecin (Boc-<strong>[86639-52-3]SN-38</strong>) as a yellow solid (0.6 g, 48% yield). |
| 48% |
With pyridine; In N,N-dimethyl-formamide; at 20℃; for 12h; |
Example 153: Synthesis of CDP-Gly-<strong>[86639-52-3]SN-38</strong> Conjugate<strong>[86639-52-3]SN-38</strong> was derivatized with the amino acid glycine at the 20-OH position as shown in Scheme 1. Briefly, 20(S)-<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (<strong>[86639-52-3]SN-38</strong>, l.Og, 2.5 mmol) was dissolved in a mixture of 70 mL dimethylformamide (DMF) and 30 mL pyridine. A solution of di-tert-butyl-dicarbonate (0.83 g, 3.8 mmol) in 10 mL DMF was added and the mixture stirred at room temperature overnight (12 hours). The solvent was removed under vacuum to yield a yellow solid and re-crystallized from boiling 2-propanol (75 mL) to yield 20(s)-10-tert-butoxycarbonyloxy-7-ethyl- camptothecin (Boc-<strong>[86639-52-3]SN-38</strong>) as a yellow solid (0.6 g, 48% yield). |
| 48% |
With pyridine; In N,N-dimethyl-formamide; at 20℃; for 12h; |
Example 6 Synthesis of CDP-Gly-<strong>[86639-52-3]SN-38</strong> Conjugate <strong>[86639-52-3]SN-38</strong> was derivatized with the amino acid glycine at the 20-OH position as shown in Scheme 1. Briefly, 20(S)-<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (<strong>[86639-52-3]SN-38</strong>, 1.0 g, 2.5 mmol) was dissolved in a mixture of 70 mL dimethylformamide (DMF) and 30 mL pyridine. A solution of di-tert-butyl-dicarbonate (0.83 g, 3.8 mmol) in 10 mL DMF was added and the mixture stirred at room temperature overnight (12 hours). The solvent was removed under vacuum to yield a yellow solid and re-crystallized from boiling 2-propanol (75 mL) to yield 20(s)-10-tert-butoxycarbonyloxy-7-ethyl-camptothecin (Boc-<strong>[86639-52-3]SN-38</strong>) as a yellow solid (0.6 g, 48% yield). |
| 48% |
With pyridine; In N,N-dimethyl-formamide; at 20℃; for 12h; |
<strong>[86639-52-3]SN-38</strong> was derivatized with the amino acid glycine at the 20-OH position as shown in Scheme VIII. Briefly, 20(S)-<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (<strong>[86639-52-3]SN-38</strong>, 1.0 g, 2.5 mmol) was dissolved in a mixture of 70 mL dimethylformamide (DMF) and 30 mL pyridine. A solution of di-tert-butyl-dicarbonate (0.83 g, 3.8 mmol) in 10 mL DMF was added and the mixture stirred at room temperature overnight (12 hours). The solvent was removed under vacuum to yield a yellow solid and re-crystallized from boiling 2-propanol (75 mL) to yield 20(s)-10-tert-butoxycarbonyloxy-7-ethyl-camptothecin (Boc-<strong>[86639-52-3]SN-38</strong>) as a yellow solid (0.6 g, 48% yield). Boc-<strong>[86639-52-3]SN-38</strong> (0.73g, 1.5 mmol), N-(tertbutoxycarbonyl)glycine (0.26g, 1.5 mmol) and 4- dimethylaminopyridine (DMAP, 0.18g, 1.5 mmol) were dissolved in anhydrous methylene chloride (30 mL) and chilled to 0C. 1,3-Diisopropylcarbodiimide (DIPC, 0.19g, 1.5 mmol) was added, the mixture stirred at 0 C for 30 minutes followed by stirring for 4 hours at room temperature. The mixture was diluted with methylene chloride to 100 mL, washed twice with an aqueous solution of 0.1N hydrochloric acid (25 mL), dried over magnesium sulfate and the solvent removed under vacuum. The resulting yellow solid was purified by flash chromatography in methylene chloride:acetone (9:1) followed by solvent removal under vacuum to yield 20-O-(N-(tert-butoxycarbonyl)glycyl)-10-tert- butyoxycarbonyloxy-7-ethylcamptothecin (diBoc-Gly-<strong>[86639-52-3]SN-38</strong>, 640 mg, 67% yield). |
| 48% |
With pyridine; In N,N-dimethyl-formamide; at 20℃; |
<strong>[86639-52-3]SN-38</strong> was derivatized with the amino acid glycine at the 20-OH position as shown in Scheme 1. Briefly, 20(S)-<strong>[86639-52-3]7-ethyl-10-hydroxycamptothecin</strong> (<strong>[86639-52-3]SN-38</strong>, 1.0g, 2.5 mmol) was dissolved in a mixture of 70 mL dimethylformamide (DMF) and 30 mL pyridine. A solution of di-tert-butyl-dicarbonate (0.83 g, 3.8 mmol) in 10 mL DMF was added and the mixture stirred at room temperature overnight (12 hours). The solvent was removed under vacuum to yield a yellow solid and re-crystallized from boiling 2-propanol (75 mL) to yield 20(s)-10-tert-butoxycarbonyloxy-7-ethyl-camptothecin (Boc-<strong>[86639-52-3]SN-38</strong>) as a yellow solid (0.6 g, 48% yield). |
| 2 g |
With pyridine; at 20℃; |
. Compound 9 (1.9 g) was dissolved into 15 ml pyridine and cooled down in an ice bath. (l3oc)20 (2.4 g) was added. The reaction was stirred at RT overnight. Volatiles were removed. The organic phase was separated and the aqueous phase was extracted with DCM, and washed with iN HC1. The combined the organic phase was dried over Na2 SO4 and concentrated to give compound 10(2.0 g) as a white solid.10107] 1H NMR (300 MHz, CDC13) 8.4-8.5 (s, 1H), 8.2-8.3(t, 1H), 7.9-8.0 (t, 1H), 7.8-7.9 (t, 1H), 7.6-7.7 (t, 1H), 7.3-7.4(t, 1H), 3.1-3.2 (t, 2H), 1.4-1.5 (s, 9H), 0.8-0.9 (m, 3H). |
| 2 g |
With pyridine; at 20℃;Cooling with ice; |
1. Compound 9 (1.9g) was dissolved into 15ml pyridine and cooled down in an ice bath. (Boc)20 (2.4g) was added. The reaction was stirred at RT overnight. Volatiles were removed .The organic phase was separated and the aqueous phase was extracted with DCM, and washed with IN HC1. The combined the organic phase was dried over Na2S04 and concentrated to give compound 10 (2.0g) as a white solid. 1H NMR (300MHz, CDC13) 8.4-8.5(s, 1H), 8.2-8.3(t, 1H), 7.9-8.0(t, 1H), 7.8-7.9(t, 1H) , 7.6-7.7(t, 1H), 7.3-7.4(t, 1H), 3.1-3.2(t, 2H), 1.4-1.5(s, 9H), 0.8-0.9(m, 3H). |
|
With pyridine; In tetrahydrofuran; dichloromethane; at 20℃; |
To a solution of <strong>[86639-52-3]SN-38</strong> (3g, 7.65 mmol) in DCM/THF (150 mL/150niL) was added (Boc)20 (2g, 9.16 mmol) and pyridine (20 mL). The suspension was stirred at room temperature until the solution turned clear. The solution was diluted with DCM (100 mL) and washed with 2N HCl (100 mLx3). The organic phase was collected, dried over Na2S04 and concentrated. The resulting crude product was used directly for the next step without purification. |
|
With pyridine; |
Under the condition of pyridine, (Boc) 2O was used according to the common method known in the art to protect the hydroxyl at site 10 of <strong>[86639-52-3]SN-38</strong>, thereby giving Compound 23-2. |
|
With pyridine; In tetrahydrofuran; dichloromethane; at 20℃; for 6h; |
SN38 (AK Scientifi, Inc., Mountain View, Calif.)was conjugated as described in literature (Bioorg Med Chem 1998, 6 (5), 55 1-62) with slight modification. 5N38 was dissolved in CH2C12/THF and treated with dit-butyl dicarbonate, and pyridine and stirred at room temperature for 6 h to selectively protect iO-hydroxy group of 5N38. The product will then be treated with boc glycine followed by DMAP, and DIPC was added dropwise at 0C. The stirring was continued for 6 hours at 0 C. and then at room temperature overnight. The reaction mixture was concentrated, treated with TFA, vortexed for 5 mm. The product was neutralized with diisopropylethylamine followed by the treatment of tert-butyldimethylsilylchioride (THDMS-Cl). The product was coupled with PEG using EDC and DMAP in DMF/DCM. The solvent was removed and purified by PD-b column to yield PEG5N38. |
|
With pyridine; In dichloromethane; at 20℃; for 36h; |
To a solution of SN38 (1 g, 2.55 mmol, 1.0 eq.) in dichloromethane (400 mL) were added pyridine (5 mL) and Boc anhydride (6.39 g, 6.371 mmol, 2.5 eq.) and the reaction mixture was stirred at room temperature for 36 h. The reaction mixture was filtered and washed with 0.01N HCl (3×). The organic phase was separated, dried (sodium sulfate) and evaporated under reduced pressure to provide compound 1 (1.26 g), which was used in the next step without further purification. |
|
With pyridine; In dichloromethane; at 20℃; for 36h; |
Example 1: Preparation of Compound 1 (0177) (0178) To a solution of SN38 (1 g, 2.55 mmol, 1.0 eq.) in dichloromethane (400 mL) were added pyridine (5 mL) and Boc anhydride (6.39 g, 6.371 mmol, 2.5 eq.) and the reaction mixture was stirred at room temperature for 36 h. The reaction mixture was filtered and washed with 0.01N HCl (3×). The organic phase was separated, dried (sodium sulfate) and evaporated under reduced pressure to provide compound 1 (1.26 g), which was used in the next step without further purification |
| 11.82 g |
With pyridine; In dichloromethane; |
To an oven-dried 1000 mL 3-neck round bottom flask fitted with a stir bar was added <strong>[86639-52-3]SN-38</strong> (6, 10 g, 25.5 mmol). Cannula transferred DCM (489 mL) to the flask. Added pyridine (525.3 mmol, 42.3 mL) and di-tert-butyl dicarbonate (Boc2O) (42.3 mL, 33.2 mmol) sequentially to the flask. Stir overnight. To work up, transferred the reaction to a 1 L recovery flask and removed the solvent on a rotary evaporator. Recrystallized the product from boiling isopropanol. Collected the solid by filtration and washed the filtrate with cold isopropanol. Blew dry the solid under argon overnight to yield the product as a light-yellow solid (11.82 g). |
|
With pyridine; In dichloromethane; at 20℃; |
Boc2O (362 mg, 1.3 eq) and pyridine (3.08 mL, 30 eq) was added tothe suspension of <strong>[86639-52-3]SN-38</strong> in DCM. The mixture was stirring at roomtemperature for overnight. The solution was washed by 1 N HCl, Sat.NaHCO3, brine, and dried with Na2SO4. The solvent was removed undervacuum to give the crude product (1.25 g, 100%).1H NMR (400 MHz,CDCl3) delta 8.22 (d, J=9.2 Hz, 1H), 7.85 (d, J=2.5 Hz, 1H), 7.67 (s,1H), 7.63 (dd, J=9.2, 2.5 Hz, 1H), 5.72 (d, J=16.3 Hz, 1H), 5.29 (d,J=16.3 Hz, 1H), 5.23 (d, J=0.8 Hz, 2H), 4.25 (s, 1H), 3.14 (q,J=7.7 Hz, 2H), 1.94-1.85 (m, 2H), 1.62 (s, 9H), 1.40 (t, J=7.7 Hz,3H), 1.02 (t, J=7.4 Hz, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +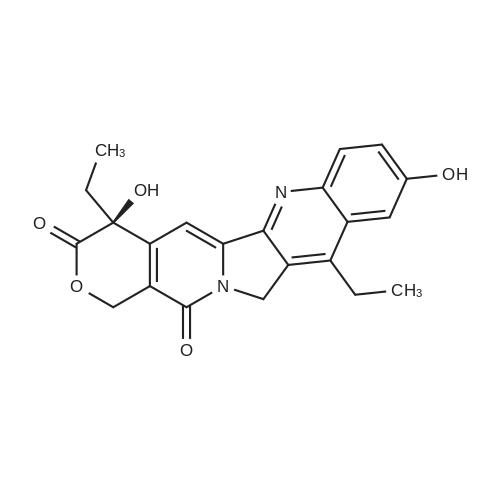

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping