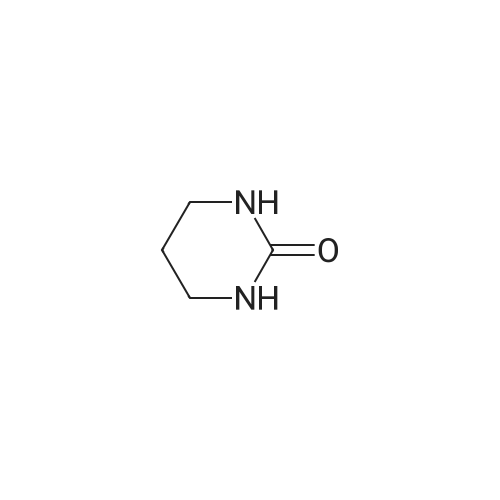
| 38% |
With sodium ethanolate; In hydrogenchloride; water; N,N-dimethyl-formamide; |
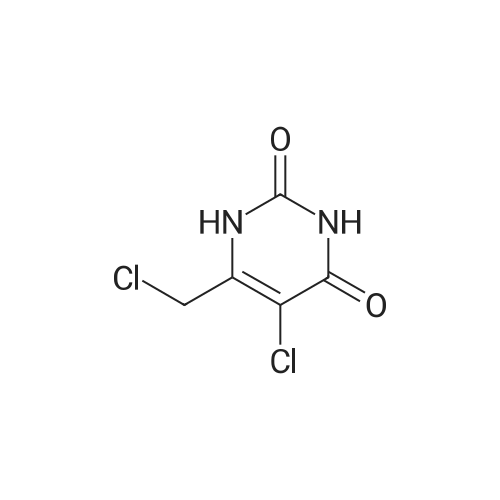
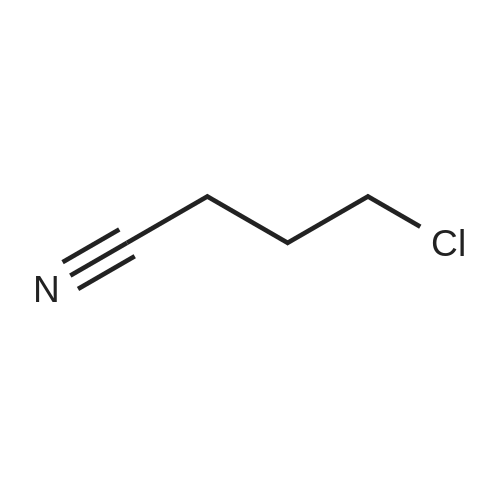
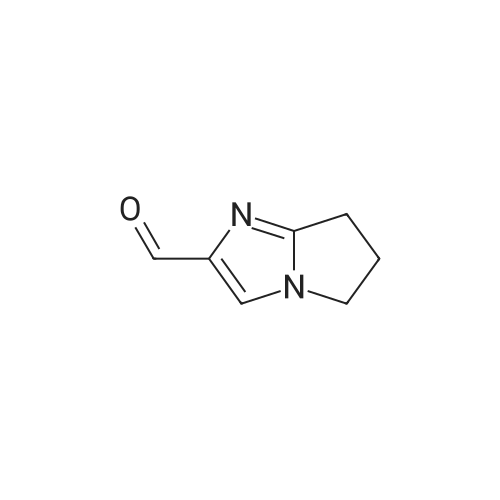
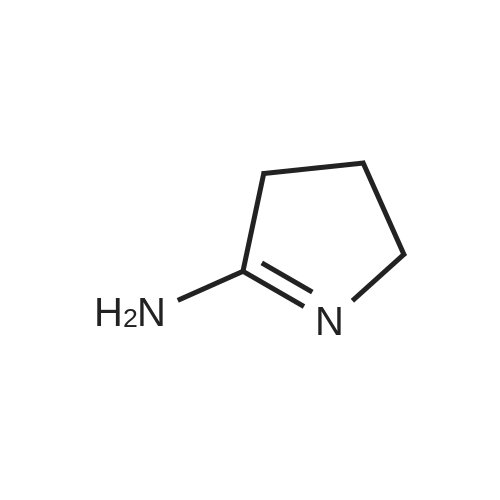
(2) 5-Chloro-6-chloromethyluracil (5.0 g), 2-iminopyrrolidine (6.14 g), and sodium ethoxide (5.24 g) were dissolved in N,N-dimethylformamide (50 ml) and the resultant solution was stirred for 14 hours at room temperature. Subsequently, precipitated crystals were collected through filtration, and the crystals were suspended in water (30 ml). The resultant suspension was neutralized with acetic acid and washed. Subsequently, insoluble matter was collected through filtration and was dissolved in 1N HCl (60 ml). Activated carbon was added to the resultant solution and the mixture was subjected to filtration. The filtrate was concentrated under reduced pressure, and the residue was washed with ethanol, followed by filtration, to thereby obtain 2.68 g of the title compound (38% yield). Melting Point: >=255 C. (decomposed) NMR spectral data (DMSO-d6) δ 2.04 (2H, quintet, J=7.6 Hz), 2.87 (2H, t, J=7.6 Hz), 3.59 (2H, t, J=7.6 Hz), 4.69 (2H, s), 9.40 (1H, s), 11.46 (1H, s), 11.73 (1H, s). |
| 38% |
With sodium ethanolate; In hydrogenchloride; water; N,N-dimethyl-formamide; |
Example 6 Synthesis of 5-chloro-6-(1-(2-iminopyrrolidinyl)-methyl)uracil hydrochloride (Compound 29) A solution of 5.0 g of 5-chloro-6-chloromethyl-uracil, 6.14 g of 2-iminopyrrolidine and 5.24 g of sodium ethoxide in N,N-dimethylformamide (50 ml) was stirred at room temperature for 14 hours. A crystallized matter was collected by filtration and then suspended in 30 ml of water. After the suspension was neutralized with acetic acid and then washed, an insoluble matter was collected by filtration and then dissolved in 60 ml of 1N hydrochloric acid. Activated carbon was added to the resultant solution, followed by filtration. The filtrate was concentrated under reduced pressure, and the residue so obtained was washed with ethanol and collected with filtration, whereby 2.68 g of the title compound were obtained (yield: 38%). |
| 38% |
With sodium ethanolate; In hydrogenchloride; water; N,N-dimethyl-formamide; |
Example 6 Synthesis of 5-chloro-6-(1-(2-iminopyrrolidinyl)-methyl)uracil hydrochloride (Compound 29) A solution of 5.0 g of <strong>[73742-45-7]5-chloro-6-chloromethyluracil</strong>, 6.14 g of 2-iminopyrrolidine and 5.24 g of sodium ethoxide in N,N-dimethylformamide (50 ml) was stirred at room temperature for 14 hours. A crystallized matter was collected by filtration and then suspended in 30 ml of water. After the suspension was neutralized with acetic acid and then washed, an insoluble matter was collected by filtration and then dissolved in 60 ml of 1 N hydrochloric acid. Activated carbon was added to the resultant solution, followed by filtration. The filtrate was concentrated under reduced pressure, and the residue so obtained was washed with ethanol and collected by filtration, whereby 2.68 g of the title compound were obtained (yield: 38%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping