| 83% |
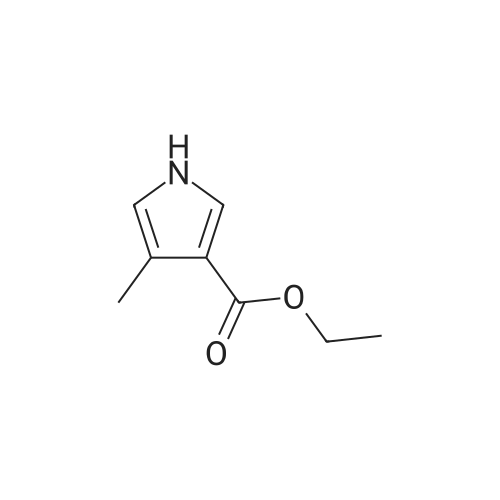
Stage #1: ethyl 5-bromo-4-methyl-1H-pyrrole-3-carboxylate With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.5h;
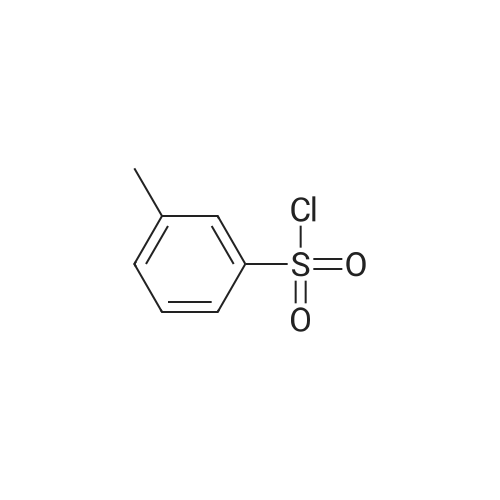
Stage #2: 3-methylphenylsulfonyl chloride In N,N-dimethyl-formamide at 20℃; for 1h; |
|
| 83% |
Stage #1: ethyl 5-bromo-4-methyl-1H-pyrrole-3-carboxylate With sodium hydride In N,N-dimethyl-formamide; mineral oil at -78 - 35℃; for 1h; Inert atmosphere;
Stage #2: 3-methylphenylsulfonyl chloride In N,N-dimethyl-formamide; mineral oil at 35℃; for 1.16667h; Cooling with ice; Inert atmosphere; |
142 Ethyl 5-bromo-4-methyl-1-[(3-methylphenyl)sulfonyl]-1H-pyrrole-3-carboxylate
Reference Example 142 Ethyl 5-bromo-4-methyl-1-[(3-methylphenyl)sulfonyl]-1H-pyrrole-3-carboxylate Under an argon atmosphere, sodium hydride (60% in oil, 452 mg) was suspended in N,N-dimethylformamide (10 mL), and a solution (10 mL) of ethyl 5-bromo-4-methyl-1H-pyrrole-3-carboxylate (2.20 g) in N,N-dimethylformamide was added dropwise at -78°C over 30 min. The mixture was stirred at room temperature for 30 min, and added dropwise to an ice-cooled solution (10 mL) of (3-methylbenzene)sulfonyl chloride (1.64 mL) in N,N-dimethylformamide over 10 min. The reaction mixture was stirred at room temperature for 1 hr, water was added, and the mixture was extracted with ethyl acetate. The extract was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=9:1→1:1), and recrystallized from diethyl ether to give the title compound as a colorless solid (yield 3.02 g, 83%). 1H-NMR (CDCl3)δ: 1.36 (3H, t, J=7.2 Hz), 2.16 (3H, s), 2.44 (3H, s), 4.29 (2H, dd, J=7.2 Hz, 14.4 Hz), 7.43-7.37 (2H, m), 7.57-7.78 (2H, m), 8.10 (1H, s). |
|
In water; N,N-dimethyl-formamide |
R.142 Ethyl 5-bromo-4-methyl-1-[(3-methylphenyl)sulfonyl]-1H-pyrrole-3-carboxylate
Reference Example 142 Ethyl 5-bromo-4-methyl-1-[(3-methylphenyl)sulfonyl]-1H-pyrrole-3-carboxylate Under an argon atmosphere, sodium hydride (60% in oil, 452 mg) was suspended in N,N-dimethylformamide (10 mL), and a solution (10 mL) of ethyl 5-bromo-4-methyl-1H-pyrrole-3-carboxylate (2.20 g) in N,N-dimethylformamide was added dropwise at -78°C over 30 min. The mixture was stirred at room temperature for 30 min, and added dropwise to an ice-cooled solution (10 mL) of (3-methylbenzene)sulfonyl chloride (1.64 mL) in N,N-dimethylformamide over 10 min. The reaction mixture was stirred at room temperature for 1 hr, water was added, and the mixture was extracted with ethyl acetate. The extract was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=9:1→1:1), and recrystallized from diethyl ether to give the title compound as a colorless solid (yield 3.02 g, 83%). 1H-NMR (CDCl3)δ: 1.36 (3H, t, J=7.2 Hz), 2.16 (3H, s), 2.44 (3H, s), 4.29 (2H, dd, J=7.2 Hz, 14.4 Hz), 7.43-7.37 (2H, m), 7.57-7.78 (2H, m), 8.10 (1H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science












 HazMat Fee +
HazMat Fee +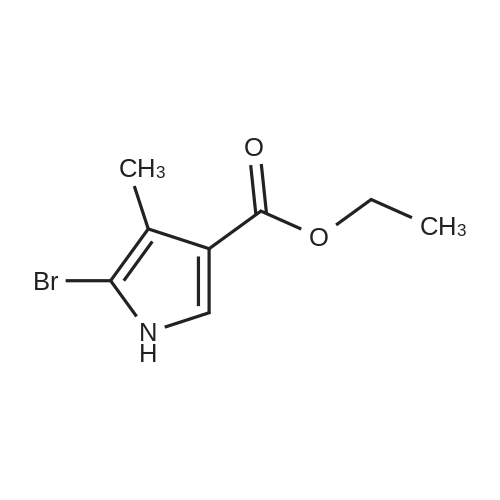

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping