Alternatived Products of [ 88597-06-2 ]
Product Details of [ 88597-06-2 ]
| CAS No. : | 88597-06-2 |
MDL No. : | MFCD00573287 |
| Formula : |
C18H17NO5S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
359.40
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 88597-06-2 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 88597-06-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 88597-06-2 ]
- 1
-
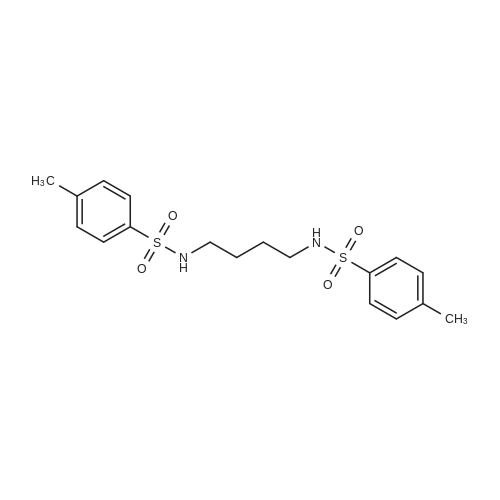 [ 15544-47-5 ]
[ 15544-47-5 ]

-
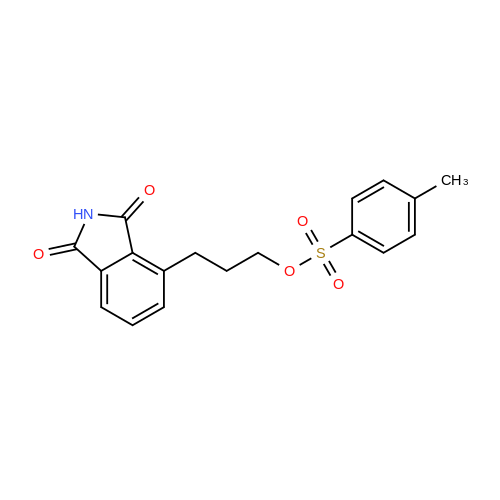 [ 88597-06-2 ]
[ 88597-06-2 ]

-
 [ 107976-61-4 ]
[ 107976-61-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 99% |
With caesium carbonate In N,N-dimethyl-formamide for 24h; Ambient temperature; |
|
| 99% |
With caesium carbonate In N,N-dimethyl-formamide for 48h; Ambient temperature; |
|
Reference:
[1]Iwata, Masaaki; Kuzuhara, Hiroyoshi
[Synthetic Communications, 1989, vol. 19, # 5and6, p. 1009 - 1014]
[2]Iwata, Masaaki; Kuzuhara, Hiroyoshi
[Bulletin of the Chemical Society of Japan, 1989, vol. 62, # 1, p. 198 - 210]
- 2
-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
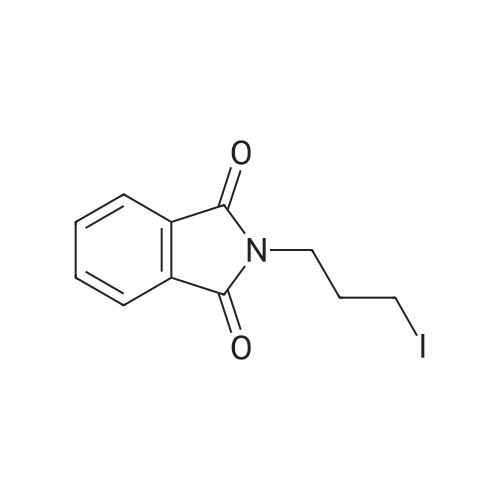 [ 5457-29-4 ]
[ 5457-29-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 88% |
With sodium iodide In acetone for 2h; Heating; |
|
| 78% |
With tetra-(n-butyl)ammonium iodide In N,N-dimethyl acetamide at 60℃; for 24h; Glovebox; Inert atmosphere; Sealed tube; |
|
|
With sodium iodide In acetone for 20h; Heating; Yield given; |
|
Reference:
[1]Martinkus; Tann; Gould
[Tetrahedron, 1983, vol. 39, # 21, p. 3493 - 3505]
[2]Cui, Ru; Sheng, Jie; Wu, Bing-Bing; Hu, Duo-Duo; Zheng, Hong-Qian; Wang, Xi-Sheng
[Chemical Communications, 2021, vol. 57, # 72, p. 9084 - 9087]
[3]Prabhakaran; Woo; Yorgey; Gould
[Journal of the American Chemical Society, 1988, vol. 110, # 17, p. 5785 - 5791]
- 3
-
 [ 15544-47-5 ]
[ 15544-47-5 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
 [ 107976-61-4 ]
[ 107976-61-4 ]

-
 [ 107976-59-0 ]
[ 107976-59-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 49%
2: 44% |
With sodium hydride In N,N-dimethyl-formamide at 85℃; |
|
- 4
-
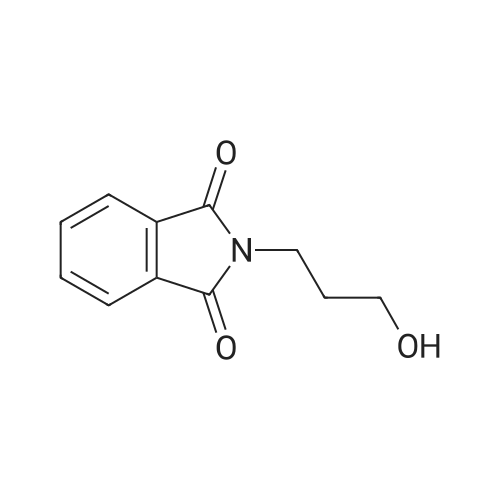 [ 883-44-3 ]
[ 883-44-3 ]

-
 [ 98-59-9 ]
[ 98-59-9 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 96% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 12.1667h; |
Step 2To a solution of 2-(3-hydroxypropyl) isoindoline-l,3-dione (1.8 g, 8.77 mmol) in CH2CI2 (30 mL, 3 mL/mmol) was added Et3N (2 mL, 13.1 mmol). After stirring for 10 min at0 C, tosylchloride (2 g, 10.5 mmol) was added and the reaction was stirred for 12 h at RT. The reaction was quenched with water and extracted with CH2CI2 (2 X50 mL). The organic extracts were washed with brine, dried over sodium sulfate and concentrated under reduced pressure. Purification by silica gel column chromatography provided 3-(l,3-dioxoisoindolin-2- yl)propyl 4-methylbenzenesulfonate (3 mg , 96%). Observed mass (M+l): 360.1. |
- 5
-
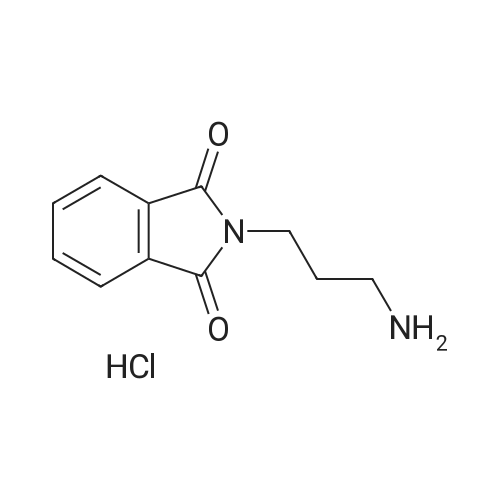 [ 121821-01-0 ]
[ 121821-01-0 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: 98 percent / Et3N, pyridine / 4 h / Ambient temperature
2: NaNO2 / acetic acid; acetic anhydride / 3 h / 5 °C
3: benzene / 20 h / 80 °C |
|
- 6
-
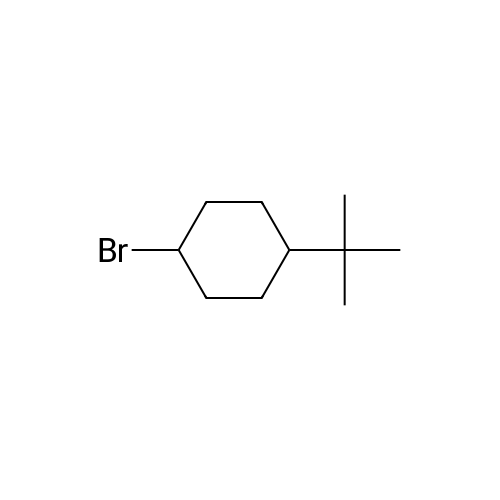 [ 7080-86-6 ]
[ 7080-86-6 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
C21H29NO2
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 48% |
With manganese; vitamin B12; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine]nickel(II) dichloride In N,N-dimethyl-formamide at 30℃; for 24h; |
|
- 7
-
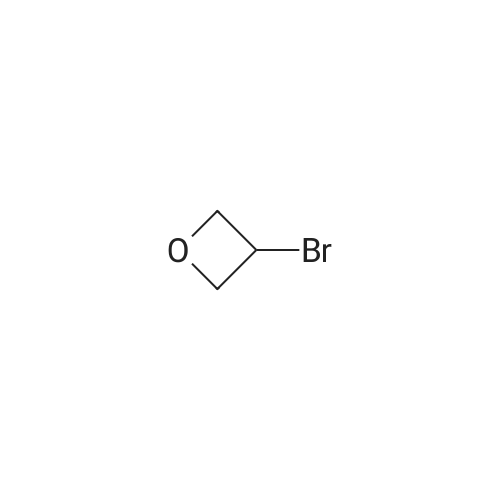 [ 39267-79-3 ]
[ 39267-79-3 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
C14H15NO3
[ No CAS ]
- 8
-
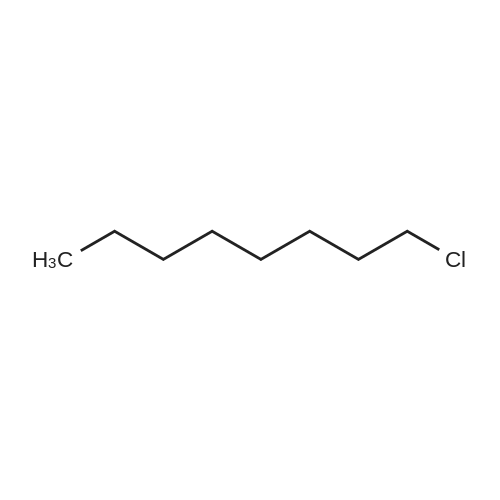 [ 111-85-3 ]
[ 111-85-3 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
2-undecylisoindoline-1,3-dione
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 60% |
With 1,4-pyrazine; manganese; vitamin B12; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine]nickel(II) dichloride In N,N-dimethyl-formamide at 30℃; for 18h; |
|
- 9
-
 [ 104-52-9 ]
[ 104-52-9 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
 [ 138619-78-0 ]
[ 138619-78-0 ]
- 10
-
 [ 85514-44-9 ]
[ 85514-44-9 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
C22H35NO3Si
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 54% |
With 1,4-pyrazine; manganese; vitamin B12; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine]nickel(II) dichloride In N,N-dimethyl-formamide at 30℃; for 36h; |
|
- 11
-
 [ 630-17-1 ]
[ 630-17-1 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
C16H21NO2
[ No CAS ]
- 12
-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
 [ 180695-79-8 ]
[ 180695-79-8 ]

-
tert-butyl 4-(3-(1,3-dioxoisoindolin-2-yl)propyl)piperidine-1-carboxylate
[ No CAS ]
- 13
-
 [ 849928-26-3 ]
[ 849928-26-3 ]

-
 [ 88597-06-2 ]
[ 88597-06-2 ]

-
C21H28N2O4
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping

















