Alternatived Products of [ 905735-40-2 ]
Product Details of [ 905735-40-2 ]
| CAS No. : | 905735-40-2 |
MDL No. : | MFCD21603873 |
| Formula : |
C7H4N2O3S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
196.18
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 905735-40-2 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 905735-40-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 905735-40-2 ]
- 1
-
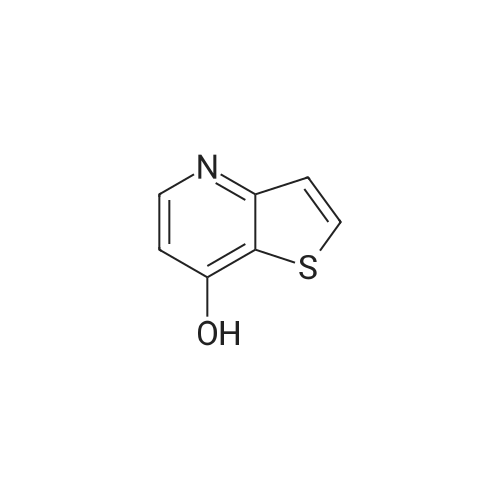 [ 107818-20-2 ]
[ 107818-20-2 ]

-
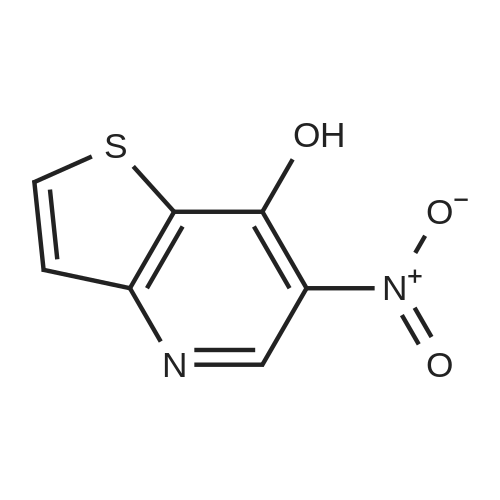 [ 905735-40-2 ]
[ 905735-40-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
Stage #1: thieno[3,2-b]pyridin-7-ol With tetrabutylammonium nitrate In dichloromethane at -5℃;
Stage #2: With trifluoroacetic anhydride In dichloromethane at -5 - 20℃; |
1.1 Step 1. 6-Nitrothieno[3,2-b]pyridin-7-ol
N,N,N-Tributylbutan-1-aminium nitrate (from Aldrich, 9.1 g, 30 mmol) dissolved in methylene chloride (100 mL) was added dropwise to a stirred solution of thieno[3,2-b]pyridin-7-ol (from Aldrich, 3.0 g, 20 mmol) in methylene chloride (100 mL) at -5° C. Trifluoroacetic anhydride (4.5 mL, 32 mmol) was added while maintaining the temperature below 0° C. The resulting mixture was then stirred at -5° C. for 30 min and at room temperature overnight. The reaction mixture was concentrated, diluted with ether, filtered. The solid collected was washed with water and then ether/methanol (MeOH) mixture (1:1), and air-dried to give the desired product (3.3 g, 85%). LCMS calculated for C7H5N2O3S (M+H)+: m/z=197.0. Found: 196.9. |
| 78% |
With tetrabutylammonium nitrate; trifluoroacetic anhydride In dichloromethane at -10 - 30℃; for 24.5h; |
5.1 6-Nitrothieno[3,2-b]pyridin-7-ol
A solution of N,N,N-tributylbutan-1-aminium nitrate (4.780 g, 15.70 mmol) in DCM (20 mL) was added to a solution of thieno[3,2-b]pyridin-7-ol (Aldrich, 1.545 g, 10.22 mmol) in DCM (15 mL) at -10° C. Trifluoroacetic anhydride (3.524 g, 16.78 mmol) was then added dropwise. After stirring at -10° C. for 30 min., the mixture was allowed to warm to room temperature and stirred for 24 h. The reaction mixture was concentrated under reduced pressure. The resulting residue was triturated with ether (50 mL), and filtered. The filter cake was washed with water (100 mL) and ether/MeOH (1:1, 80 mL), and was then dried to give the sub-title compound as a yellow solid (1.56 g, 78%). LCMS calc. for C7H5N2O3S (M+H)+: m/z=197.0. found 197.0. |
| 74% |
With nitric acid In propionic acid at 110℃; for 1.03333h; Heating / reflux; |
A2.A2.1
A2.1: 6-Nitro-7-hydroxythieno[3,2-b]pyridine; Commercially available 7-hydroxythieno[3,2-b]pyridine (9.9 g, 65.5 mmol) was dissolved in propionic acid (200 mL) and heated to 110° C., behind a bomb shield. 4.85 mL of fuming nitric acid (90%) was added over 2 minutes, during which time a copius precipitate formed. The thick suspension was brought to reflux (oil bath temperature 150° C.) for 1 h during which time an orange gas evolved (Caution: procedure should only be performed in a well ventilated hood). The reaction mixture was allowed to cool to room temperature and 200 mL of diethyl ether was added. The product was collected by filtration, washed with 400 mL of water, 300 mL of 1:1 diethyl ether/methanol, and dried to yield 9.5 g, (74%) of A2.1 as a tan powder. M.S. 197 (M+H)+ 100%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping



