Alternatived Products of [ 95068-02-3 ]
Product Details of [ 95068-02-3 ]
| CAS No. : | 95068-02-3 |
MDL No. : | MFCD14686971 |
| Formula : |
C8H8BrFO
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
219.05
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 95068-02-3 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 95068-02-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 95068-02-3 ]
- 1
-
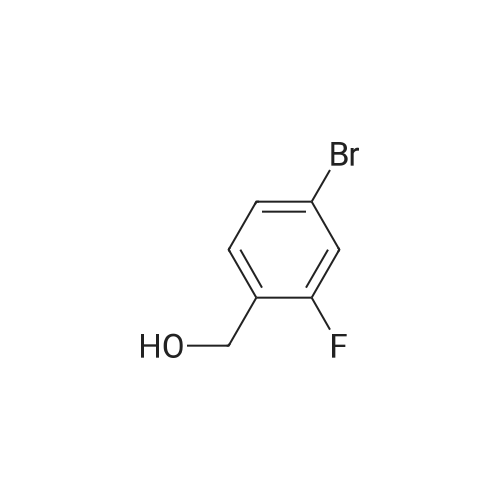 [ 188582-62-9 ]
[ 188582-62-9 ]

-
 [ 74-88-4 ]
[ 74-88-4 ]

-
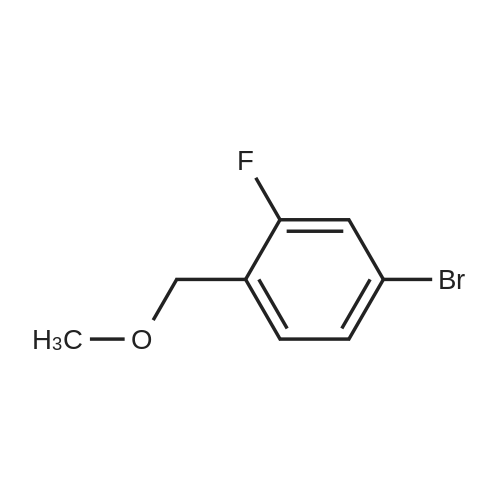 [ 95068-02-3 ]
[ 95068-02-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: 4-bromo-2-fluorobenzyl alcohol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 0.75h;
Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; |
17
The title compound was prepared according to General Method 8. To a suspension of NaH (50-60 % mineral oil , 8 g, 0.33 mol) in Dry DMF ( 200 mL ) at 0 0C was added (4-bromo-2-fluorophenyl)methanol ( 27 g , 0.1317 mol). The reaction mixture was stirred for 15 min at 0 0C and then warmed to and stirred at RT for 30 min. The reaction mixture was again cooled to 0 0C and MeI (15 mL, 0.1975 mol) was added drop wise. After addition was complete, the reaction mixture was warmed to room temperature and stirred for Ih. Ice- water was added and the product was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulphate, and concentrated under reduced pressure to obtained 4-bromo-2- fluoro-l-(methoxymethyl)benzene (24 g). |
| 14.8 g |
Stage #1: 4-bromo-2-fluorobenzyl alcohol With sodium hydride In tetrahydrofuran at 0 - 20℃; for 2h;
Stage #2: methyl iodide In tetrahydrofuran for 3h; |
1-2
Sodium hydride and THF (20 mL) were mixed and cooled to 0°C. Under cooling, 2-fluoro-4-bromobenzbenzyl alcohol (16.8) obtained in (1-2) was obtained.g) and THF (80 mL) were slowly added, and the mixture was returned to room temperature and stirred for 2 hours. Subsequently, iodomethane (23.3 g) was slowly added, and the mixture was further stirred for 3 hours.The reaction solution was cooled to 0 ° C., water (50 mL) was slowly added, ethyl acetate (200 mL) was added for liquid separation, and the organic layer was washed with saturated brine (100 mL). After anhydrous sodium sulfate was added and dried, sodium sulfate was removed by filtration. The organic solvent was concentrated under reduced pressure. The obtained residue was dissolved in hexane and purified by silica gel column chromatography to give 14.8 g of 1-Fluoro-3-bromo-5-methoxymethylbenzene was obtained as a clear liquid. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping





