Alternatived Products of [ 99065-34-6 ]
Product Details of [ 99065-34-6 ]
| CAS No. : | 99065-34-6 |
MDL No. : | MFCD08234752 |
| Formula : |
C9H17NO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
171.24
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 99065-34-6 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 99065-34-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 99065-34-6 ]
- 1
-
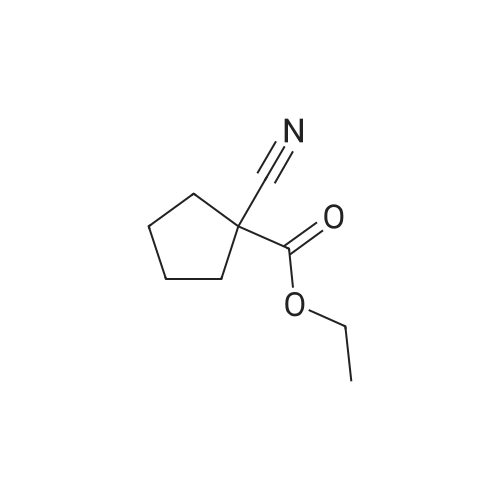 [ 28247-14-5 ]
[ 28247-14-5 ]

-
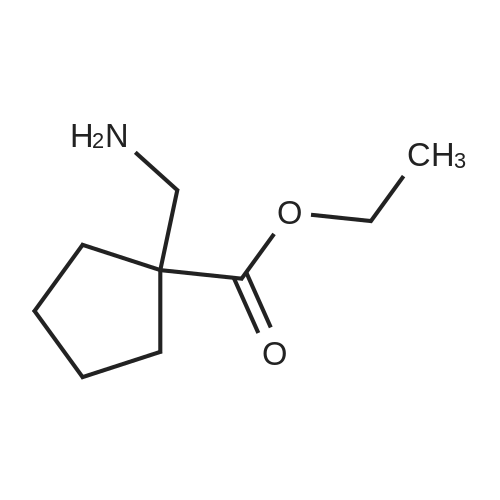 [ 99065-34-6 ]
[ 99065-34-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogen In ethanol |
|
|
With hydrogen |
|
|
With ammonia In aluminum nickel; ethanol |
39.a N-(2-Ethoxycarbonyl-2,2-tetramethylen-ethyl)-N-pentanoyl-N-[2'-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]-amine
a) Ethyl 1-aminomethyl-cyclopentane-1-carboxylate is obtained by hydrogenating 33 g of ethyl 1-cyanocyclopentane-1-carboxylate (Alfred Bader Chemicals) in the presence of 10 g of Raney nickel, at 45° C. and under normal pressure in 330 ml of ethanol which contains about 4% ammonia. After filtering off the catalyst and removing the solvent in vacuo, the product is obtained by distillation, b.p. 71°-74° C. at 0.75 mbar. |
|
With ammonia In aluminum nickel; ethanol |
2.a EXAMPLE 2
a) 1-Aminomethylcyclopentane-1-carboxylic acid ethyl ester is obtained by hydrogenating 33 g of 1-cyanocyclopentane-1-carboxylic acid ethyl ester (Alfred Bader Chemicals) in 330 ml of ethanol, which contains approximately 4% ammonia, in the presence of 10 g of Raney nickel at 45° and under normal pressure. After filtering off from the catalyst and removing the solvent in vacuo, the product is obtained by distillation, b.p. 71°-74° at 0.75 mbar. |

Reference:
[1]Testa,E. et al.
[Justus Liebigs Annalen der Chemie, 1961, vol. 639, p. 166 - 180]
[2]Fotouhi, Nader; Joshi, Pramod; Fry, David; Cook, Charles; Tilley, Jefferson W.; Kaplan, Gerry; Hanglow, Angela; Rowan, Karen; Schwinge, Virginia; Wolitzky, Barry
[Bioorganic and Medicinal Chemistry Letters, 2000, vol. 10, # 11, p. 1171 - 1173]
[3]Current Patent Assignee: Novartis (w/o Sandoz); NOVARTIS AG - US5399578, 1995, A
[4]Current Patent Assignee: Novartis (w/o Sandoz); NOVARTIS AG - US5506240, 1996, A
- 2
-
 [ 99065-34-6 ]
[ 99065-34-6 ]

-
 [ 204514-22-7 ]
[ 204514-22-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
2: NaOH / dioxane |
|
Reference:
[1]Fotouhi, Nader; Joshi, Pramod; Fry, David; Cook, Charles; Tilley, Jefferson W.; Kaplan, Gerry; Hanglow, Angela; Rowan, Karen; Schwinge, Virginia; Wolitzky, Barry
[Bioorganic and Medicinal Chemistry Letters, 2000, vol. 10, # 11, p. 1171 - 1173]
- 3
-
CH2 Cl2 -MeOH
[ No CAS ]

-
 [ 99065-34-6 ]
[ 99065-34-6 ]

-
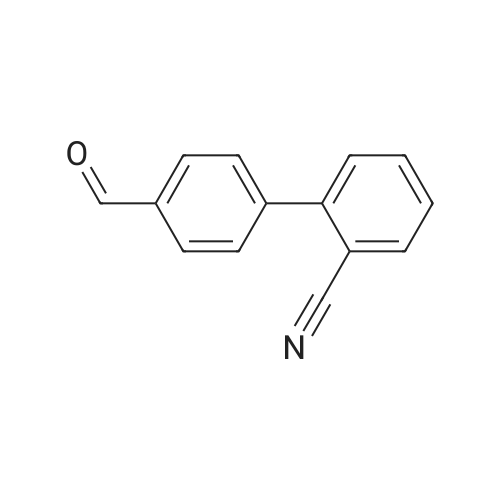 [ 135689-93-9 ]
[ 135689-93-9 ]

-
 [ 137864-26-7 ]
[ 137864-26-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
39.b N-(2-Ethoxycarbonyl-2,2-tetramethylen-ethyl)-N-pentanoyl-N-[2'-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]-amine
b) Ethyl N-[(2'-cyanobiphenyl-4-yl)methyl]-1-aminomethylcyclopentane-1-carboxylate is obtained from 4.15 g of 2'-cyanobiphenyl-4-carbaldehyde and 4.15 g of ethyl 1-aminomethylcyclopentane-1-carboxylate analogously to Example 1b) and purified on silica gel 60 (40-63 μm) using CH2 Cl2 -MeOH 99.5:0.5, Rf =0.38 (system N6). |
- 4
-
 [ 28247-14-5 ]
[ 28247-14-5 ]

-
 [ 96042-30-7 ]
[ 96042-30-7 ]

-
 [ 99065-34-6 ]
[ 99065-34-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With ammonium hydroxide; ammonia In methanol; ethanol |
29.B B
B Ethyl 1-aminomethylcyclopentanecarboxylate This compound is prepared by the catalytic hydrogenation of ethyl 1-cyanocyclopentanecarboxylate. 20 g of ethyl 1-cyanocyclopentanecarboxylate are placed in 200 ml of a 10% solution of ammonia in ethanol and hydrogenated at 60° C. under a pressure of 100 bar in the presence of rhodium-on-alumina for 72 hours. After filtration on cellite and evaporation, the residue is chromatographed on silica using a DCM/methanol/20% aqueous ammonia mixture (98/2/0.5; v/v/v) as the eluent. m=12.8 g. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









