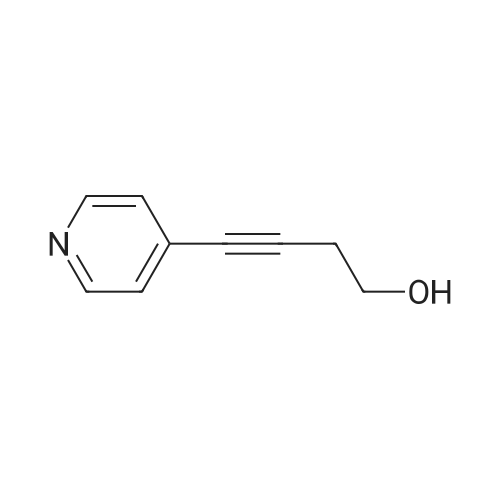
| 51% |
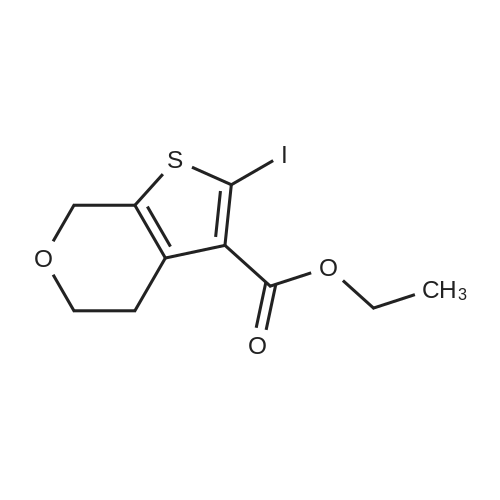
Stage #1: 2-iodo-5,7-dihydro-4H-thieno[2,3-c]pyran-3-carboxylic acid ethyl ester With copper (I) iodide; palladium 10% on activated carbon; triethylamine; triphenylphosphine In ethanol for 0.25h; Inert atmosphere;
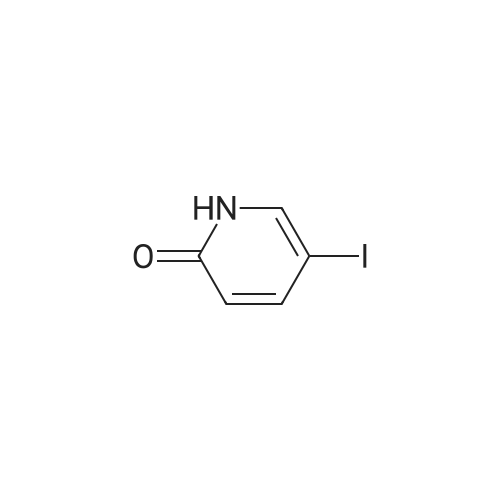
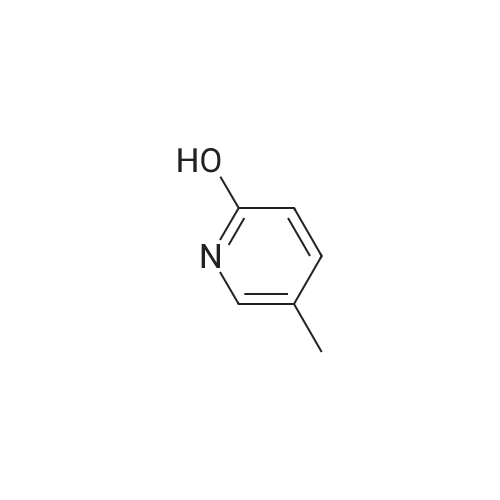
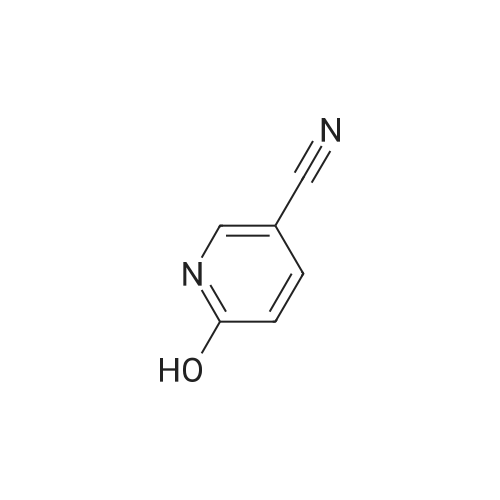
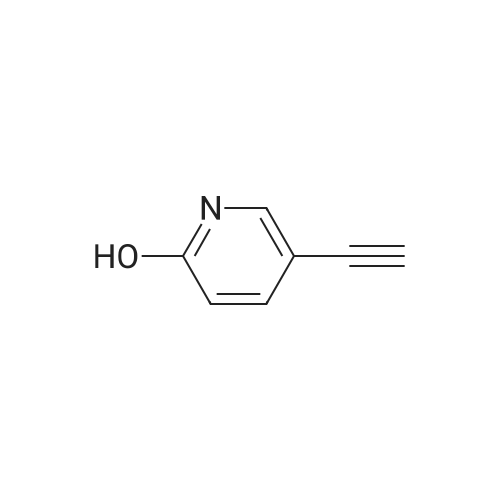
Stage #2: 5-ethynylpyridin-2(1H)-one In ethanol at 60℃; for 12h; Inert atmosphere; |
1-6 Examples 1-6, Preparation of (S)-4- (1- (2-((6-oxo-1,6-dihydropyridin-3-yl)ethynyl)-4,7-dihydro-5H- thieno [2,3-c] pyran-3-carboxamido)ethyl)benzoic acid (YJ137)
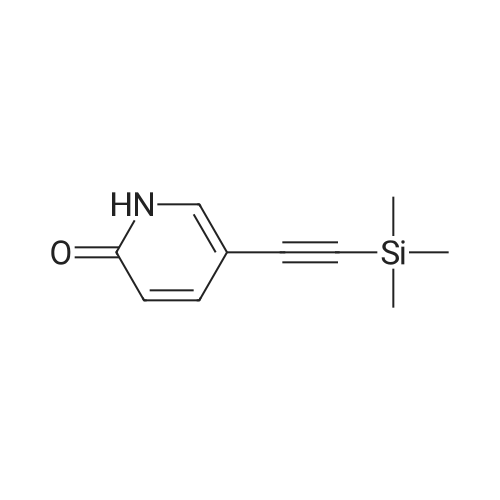
Take 2-iodo-5,7-dihydro-4H-thieno [2,3-c] pyran-3-carboxylic acid ethyl ester (160mg, 0.48mmol), 10% Pd / C (5mg, 0.048mmol ),PPh3 (5mg, 0.02mmol), CuI (9mg, 0.048mmol) and triethylamine (0.13mL, 0.93mmol) were added to 10.0mL ethanol,The mixture was stirred for 15 min under the protection of nitrogen.Then 5-acetylene-2 (1H) -pyridone (85 mg, 0.71 mmol) was added to the reaction solution,The reaction was continued to be stirred at 60 ° C for 12 h. After the reaction was completed, the reaction solution was extracted with water and ethyl acetate. The organic phase was evaporated to dryness and purified using column chromatography to obtain a white solid.That is 2-((6-oxo-1,6-dihydropyridin-3-yl) ethynyl) -4,7-dihydro-5H-thieno [2,3-c]Pyran-3-carboxylic acid ethyl ester (80 mg, 51% yield).2-((6-oxo-1,6-dihydropyridin-3-yl) ethynyl) -4,7-dihydro-5H-thieno [2,3-c] pyran-3-carboxy Ethyl acetate (80mg, 0.25mmol),3.0mL THF, 3.0mL methanol,1.0mL of water and lithium hydroxide monohydrate (21mg, 0.5mmol)Mix together and stir the reaction at 68 ° C for 3h. After the reaction, the reaction solution was made acidic with 2M HCl, and then extracted with ethyl acetate and water. The organic phase was evaporated to dryness and purified by column chromatography to obtain a white solid.I.e. 2-((6-oxo-1,6-dihydropyridin-3-yl) ethynyl) -4,7-dihydro-5H-thieno [2,3-c] pyran-3-carboxy Acid (70 mg, yield 93%).2-((6-oxo-1,6-dihydropyridin-3-yl) ethynyl) -4,7-dihydro-5H-thieno [2,3-c] pyran-3-carboxy Acid (70mg, 0.23mmol), (S) -4- (1-aminoethyl) benzoic acid methyl ester (48mg, 0.26mmol), HATU (137mg, 0.36mmol) and DIEA (65mg, 0.50mmol) were dissolved in 2.0 In mL DMF, stir at room temperature for 6h. After the reaction is completed, the reaction solution is extracted with ethyl acetate and water. The upper organic phase is evaporated to dryness and purified by column chromatography to obtain a white solid, that is,(S) -4- (1- (2-((6-oxo-1,6-dihydropyridin-3-yl) ethynyl) -4,7-dihydro-5H-thieno [2,3 -c] methyl pyran-3-carboxamido) ethyl) benzoate (59 mg, yield 55%).Take (S) -4- (1- (2-((6-oxo-1,6-dihydropyridin-3-yl) ethynyl) -4,7-dihydro-5H-thieno [2, 3-c] pyran-3-carboxamido) ethyl) benzoate (59 mg, 0.13 mmol)Dissolved in a solution consisting of 3.0mL of THF, 3.0mL of methanol, and 1.0mL of water, and then added lithium hydroxide monohydrate (10mg, 0.24mmol). The reaction solution was stirred at 68 ° C for 3h. After the reaction was completed, 2M was used. The reaction solution was made acidic with HCl, extracted with water and ethyl acetate, and the organic phase was evaporated to dryness, and purified by column chromatography to obtain a white solid., That is, the final product YJ137 (39 mg, yield 66%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping